Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Helical Contributions Mediate Light-Activated Conformational Change in the LOV2 Domain of Avena sativa Phototropin 1.
ACS Omega ( IF 3.7 ) Pub Date : 2019-01-15 , DOI: 10.1021/acsomega.8b02872
Josiah P Zayner 1 , Tilo Mathes 2 , Tobin R Sosnick 1, 3 , John T M Kennis 2
ACS Omega ( IF 3.7 ) Pub Date : 2019-01-15 , DOI: 10.1021/acsomega.8b02872
Josiah P Zayner 1 , Tilo Mathes 2 , Tobin R Sosnick 1, 3 , John T M Kennis 2
Affiliation
|
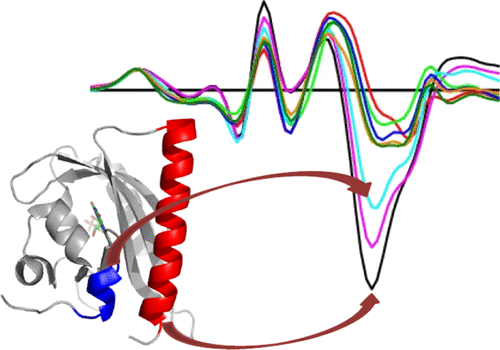
Algae, plants, bacteria, and fungi contain flavin-binding light-oxygen-voltage (LOV) domains that function as blue light sensors to control cellular responses to light. In the second LOV domain of phototropins, called LOV2 domains, blue light illumination leads to covalent bond formation between protein and flavin that induces the dissociation and unfolding of a C-terminally attached α helix (Jα) and the N-terminal helix (A'α). To date, the majority of studies on these domains have focused on versions that contain truncations in the termini, which creates difficulties when extrapolating to the much larger proteins that contain these domains. Here, we study the influence of deletions and extensions of the A'α helix of the LOV2 domain of Avena sativa phototropin 1 (AsLOV2) on the light-triggered structural response of the protein by Fourier-transform infrared difference spectroscopy. Deletion of the A'α helix abolishes the light-induced unfolding of Jα, whereas extensions of the A'α helix lead to an attenuated structural change of Jα. These results are different from shorter constructs, indicating that the conformational changes in full-length phototropin LOV domains might not be as large as previously assumed, and that the well-characterized full unfolding of the Jα helix in AsLOV2 with short A'α helices may be considered a truncation artifact. It also suggests that the N- and C-terminal helices of phot-LOV2 domains are necessary for allosteric regulation of the phototropin kinase domain and may provide a basis for signal integration of LOV1 and LOV2 domains in phototropins.
中文翻译:

螺旋贡献介导燕麦趋光素 1 的 LOV2 结构域的光激活构象变化。
藻类、植物、细菌和真菌含有黄素结合光氧电压 (LOV) 结构域,可充当蓝光传感器来控制细胞对光的反应。在向光素的第二个 LOV 结构域(称为 LOV2 结构域)中,蓝光照射导致蛋白质和黄素之间形成共价键,从而诱导 C 端连接的 α 螺旋 (Jα) 和 N 端螺旋 (A') 解离和展开。 α)。迄今为止,关于这些结构域的大多数研究都集中在末端包含截断的版本,这在推断包含这些结构域的更大蛋白质时造成了困难。在这里,我们通过傅里叶变换红外差异光谱研究了燕麦向光素 1 (AsLOV2) 的 LOV2 结构域的 A'α 螺旋的缺失和延伸对该蛋白的光触发结构响应的影响。 A'α螺旋的缺失消除了光诱导的Jα展开,而A'α螺旋的延伸导致Jα的结构变化减弱。这些结果与较短的构建体不同,表明全长向光素 LOV 结构域的构象变化可能不像之前假设的那么大,并且具有短 A'α 螺旋的 AsLOV2 中 Jα 螺旋的充分展开特征可能是被视为截断伪影。它还表明 phot-LOV2 结构域的 N 端和 C 端螺旋对于向光素激酶结构域的变构调节是必需的,并且可能为向光素中 LOV1 和 LOV2 结构域的信号整合提供基础。
更新日期:2019-01-15
中文翻译:

螺旋贡献介导燕麦趋光素 1 的 LOV2 结构域的光激活构象变化。
藻类、植物、细菌和真菌含有黄素结合光氧电压 (LOV) 结构域,可充当蓝光传感器来控制细胞对光的反应。在向光素的第二个 LOV 结构域(称为 LOV2 结构域)中,蓝光照射导致蛋白质和黄素之间形成共价键,从而诱导 C 端连接的 α 螺旋 (Jα) 和 N 端螺旋 (A') 解离和展开。 α)。迄今为止,关于这些结构域的大多数研究都集中在末端包含截断的版本,这在推断包含这些结构域的更大蛋白质时造成了困难。在这里,我们通过傅里叶变换红外差异光谱研究了燕麦向光素 1 (AsLOV2) 的 LOV2 结构域的 A'α 螺旋的缺失和延伸对该蛋白的光触发结构响应的影响。 A'α螺旋的缺失消除了光诱导的Jα展开,而A'α螺旋的延伸导致Jα的结构变化减弱。这些结果与较短的构建体不同,表明全长向光素 LOV 结构域的构象变化可能不像之前假设的那么大,并且具有短 A'α 螺旋的 AsLOV2 中 Jα 螺旋的充分展开特征可能是被视为截断伪影。它还表明 phot-LOV2 结构域的 N 端和 C 端螺旋对于向光素激酶结构域的变构调节是必需的,并且可能为向光素中 LOV1 和 LOV2 结构域的信号整合提供基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号