当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction between the Rev1 C-Terminal Domain and the PolD3 Subunit of Polζ Suggests a Mechanism of Polymerase Exchange upon Rev1/Polζ-Dependent Translesion Synthesis
Biochemistry ( IF 2.9 ) Pub Date : 2016-03-24 00:00:00 , DOI: 10.1021/acs.biochem.5b01282
Yulia Pustovalova 1 , Mariana T. Q. Magalhães 1 , Sanjay D’Souza 2 , Alessandro A. Rizzo 1 , George Korza 1 , Graham C. Walker 2 , Dmitry M. Korzhnev 1
Biochemistry ( IF 2.9 ) Pub Date : 2016-03-24 00:00:00 , DOI: 10.1021/acs.biochem.5b01282
Yulia Pustovalova 1 , Mariana T. Q. Magalhães 1 , Sanjay D’Souza 2 , Alessandro A. Rizzo 1 , George Korza 1 , Graham C. Walker 2 , Dmitry M. Korzhnev 1
Affiliation
|
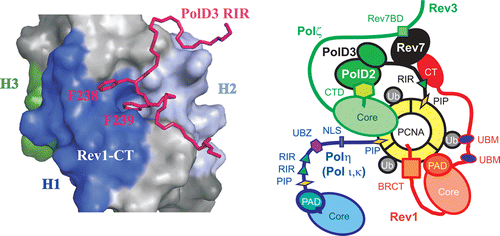
Translesion synthesis (TLS) is a mutagenic branch of cellular DNA damage tolerance that enables bypass replication over DNA lesions carried out by specialized low-fidelity DNA polymerases. The replicative bypass of most types of DNA damage is performed in a two-step process of Rev1/Polζ-dependent TLS. In the first step, a Y-family TLS enzyme, typically Polη, Polι, or Polκ, inserts a nucleotide across a DNA lesion. In the second step, a four-subunit B-family DNA polymerase Polζ (Rev3/Rev7/PolD2/PolD3 complex) extends the distorted DNA primer-template. The coordinated action of error-prone TLS enzymes is regulated through their interactions with the two scaffold proteins, the sliding clamp PCNA and the TLS polymerase Rev1. Rev1 interactions with all other TLS enzymes are mediated by its C-terminal domain (Rev1-CT), which can simultaneously bind the Rev7 subunit of Polζ and Rev1-interacting regions (RIRs) from Polη, Polι, or Polκ. In this work, we identified a previously unknown RIR motif in the C-terminal part of PolD3 subunit of Polζ whose interaction with the Rev1-CT is among the tightest mediated by RIR motifs. Three-dimensional structure of the Rev1-CT/PolD3-RIR complex determined by NMR spectroscopy revealed a structural basis for the relatively high affinity of this interaction. The unexpected discovery of PolD3-RIR motif suggests a mechanism of “inserter” to “extender” DNA polymerase switch upon Rev1/Polζ-dependent TLS, in which the PolD3-RIR binding to the Rev1-CT (i) helps displace the “inserter” Polη, Polι, or Polκ from its complex with Rev1, and (ii) facilitates assembly of the four-subunit “extender” Polζ through simultaneous interaction of Rev1-CT with Rev7 and PolD3 subunits.
中文翻译:

Rev1 C末端域和Polζ的PolD3亚基之间的相互作用表明,Rev1 /Polζ依赖的转运合成后聚合酶交换的机制。
跨病变合成(TLS)是细胞DNA损伤耐受性的诱变分支,可通过专门的低保真DNA聚合酶对DNA损伤进行旁路复制。大多数类型的DNA损伤的复制旁路均通过Rev1 /Polζ依赖性TLS的两步过程完成。第一步,Y族TLS酶(通常为Polη,PolI或Polκ)在DNA损伤中插入一个核苷酸。在第二步中,四亚基B族DNA聚合酶Polζ(Rev3 / Rev7 / PolD2 / PolD3复合物)扩展了扭曲的DNA引物模板。容易出错的TLS酶通过与两个支架蛋白,滑动钳位PCNA和TLS聚合酶Rev1的相互作用来调节其协同作用。Rev1与所有其他TLS酶的相互作用是由其C末端结构域(Rev1-CT)介导的,它们可以同时结合Polζ和来自Polη,PolI或Polκ的Rev1相互作用区域(RIR)的Rev7亚基。在这项工作中,我们在Polζ的PolD3亚基的C末端部分鉴定了一个以前未知的RIR基序,该基团与Rev1-CT的相互作用是由RIR基序介导的最紧密的序列之一。通过NMR光谱确定的Rev1-CT / PolD3-RIR复合物的三维结构揭示了这种相互作用相对较高的亲和力的结构基础。PolD3-RIR基序的意外发现表明,在依赖Rev1 /Polζ的TLS上,“插入物”可“延长” DNA聚合酶转换的机制,其中PolD3-RIR与Rev1-CT的结合(i)有助于置换“插入物” ”Polη,Polι或Polκ与Rev1的复合体,
更新日期:2016-03-24
中文翻译:

Rev1 C末端域和Polζ的PolD3亚基之间的相互作用表明,Rev1 /Polζ依赖的转运合成后聚合酶交换的机制。
跨病变合成(TLS)是细胞DNA损伤耐受性的诱变分支,可通过专门的低保真DNA聚合酶对DNA损伤进行旁路复制。大多数类型的DNA损伤的复制旁路均通过Rev1 /Polζ依赖性TLS的两步过程完成。第一步,Y族TLS酶(通常为Polη,PolI或Polκ)在DNA损伤中插入一个核苷酸。在第二步中,四亚基B族DNA聚合酶Polζ(Rev3 / Rev7 / PolD2 / PolD3复合物)扩展了扭曲的DNA引物模板。容易出错的TLS酶通过与两个支架蛋白,滑动钳位PCNA和TLS聚合酶Rev1的相互作用来调节其协同作用。Rev1与所有其他TLS酶的相互作用是由其C末端结构域(Rev1-CT)介导的,它们可以同时结合Polζ和来自Polη,PolI或Polκ的Rev1相互作用区域(RIR)的Rev7亚基。在这项工作中,我们在Polζ的PolD3亚基的C末端部分鉴定了一个以前未知的RIR基序,该基团与Rev1-CT的相互作用是由RIR基序介导的最紧密的序列之一。通过NMR光谱确定的Rev1-CT / PolD3-RIR复合物的三维结构揭示了这种相互作用相对较高的亲和力的结构基础。PolD3-RIR基序的意外发现表明,在依赖Rev1 /Polζ的TLS上,“插入物”可“延长” DNA聚合酶转换的机制,其中PolD3-RIR与Rev1-CT的结合(i)有助于置换“插入物” ”Polη,Polι或Polκ与Rev1的复合体,







































 京公网安备 11010802027423号
京公网安备 11010802027423号