当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient synthesis of chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides via Rh-catalyzed hydrogenation†
Chemical Science ( IF 7.6 ) Pub Date : 2019-01-15 00:00:00 , DOI: 10.1039/c8sc05397a Gongyi Liu 1, 2, 3, 4, 5 , Heng Zhang 1, 2, 3, 4, 5 , Yi Huang 1, 2, 3, 4, 5 , Zhengyu Han 1, 2, 3, 4, 5 , Gang Liu 1, 2, 3, 4, 5 , Yuanhua Liu 1, 2, 3, 4, 5 , Xiu-Qin Dong 1, 2, 3, 4, 5 , Xumu Zhang 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2019-01-15 00:00:00 , DOI: 10.1039/c8sc05397a Gongyi Liu 1, 2, 3, 4, 5 , Heng Zhang 1, 2, 3, 4, 5 , Yi Huang 1, 2, 3, 4, 5 , Zhengyu Han 1, 2, 3, 4, 5 , Gang Liu 1, 2, 3, 4, 5 , Yuanhua Liu 1, 2, 3, 4, 5 , Xiu-Qin Dong 1, 2, 3, 4, 5 , Xumu Zhang 1, 2, 3, 4, 5
Affiliation
|
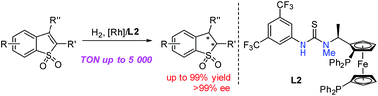
Rh-catalyzed asymmetric hydrogenation of prochiral substituted benzo[b]thiophene 1,1-dioxides was successfully developed, affording various chiral 2,3-dihydrobenzo[b]thiophene 1,1-dioxides with high yields and excellent enantioselectivities (up to 99% yield and >99% ee). In particular, for challenging substrates, such as aryl substituted substrates with sterically hindered groups and alkyl substituted substrates, the reaction proceeded smoothly in our catalytic system with excellent results. The gram-scale asymmetric hydrogenation proceeded well with 99% yield and 99% ee in the presence of 0.02 mol% (S/C = 5000) catalyst loading. The possible hydrogen-bonding interaction between the substrate and the ligand may play an important role in achieving high reactivity and excellent enantioselectivity.
中文翻译:

手性2,3-二氢-苯并[高效合成b ]噻吩-1,1-二氧化物通过铑催化的加氢†
成功开发了铑催化的前手性取代苯并[ b ]噻吩1,1-二氧化物的不对称氢化反应,提供了各种手性2,3-二氢苯并[ b]]噻吩1,1-二氧化物,具有高收率和出色的对映选择性(高达99%收率和> 99%ee)。尤其是,对于具有挑战性的底物,例如具有空间位阻基团的芳基取代的底物和烷基取代的底物,反应在我们的催化体系中能顺利进行,并获得优异的结果。在催化剂负载为0.02摩尔%(S / C = 5000)的情况下,克级不对称氢化反应进展顺利,产率为99%,ee为99%。底物和配体之间可能的氢键相互作用可能在实现高反应性和出色的对映选择性中起重要作用。
更新日期:2019-01-15
中文翻译:

手性2,3-二氢-苯并[高效合成b ]噻吩-1,1-二氧化物通过铑催化的加氢†
成功开发了铑催化的前手性取代苯并[ b ]噻吩1,1-二氧化物的不对称氢化反应,提供了各种手性2,3-二氢苯并[ b]]噻吩1,1-二氧化物,具有高收率和出色的对映选择性(高达99%收率和> 99%ee)。尤其是,对于具有挑战性的底物,例如具有空间位阻基团的芳基取代的底物和烷基取代的底物,反应在我们的催化体系中能顺利进行,并获得优异的结果。在催化剂负载为0.02摩尔%(S / C = 5000)的情况下,克级不对称氢化反应进展顺利,产率为99%,ee为99%。底物和配体之间可能的氢键相互作用可能在实现高反应性和出色的对映选择性中起重要作用。






































 京公网安备 11010802027423号
京公网安备 11010802027423号