当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetrathiomolybdate induces dimerization of the metal-binding domain of ATPase and inhibits platination of the protein.
Nature Communications ( IF 14.7 ) Pub Date : 2019-01-14 , DOI: 10.1038/s41467-018-08102-z
Tiantian Fang 1 , Wanbiao Chen 2 , Yaping Sheng 1 , Siming Yuan 1 , Qiaowei Tang 3 , Gongyu Li 1 , Guangming Huang 1 , Jihu Su 3 , Xuan Zhang 2 , Jianye Zang 2 , Yangzhong Liu 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-01-14 , DOI: 10.1038/s41467-018-08102-z
Tiantian Fang 1 , Wanbiao Chen 2 , Yaping Sheng 1 , Siming Yuan 1 , Qiaowei Tang 3 , Gongyu Li 1 , Guangming Huang 1 , Jihu Su 3 , Xuan Zhang 2 , Jianye Zang 2 , Yangzhong Liu 1
Affiliation
|
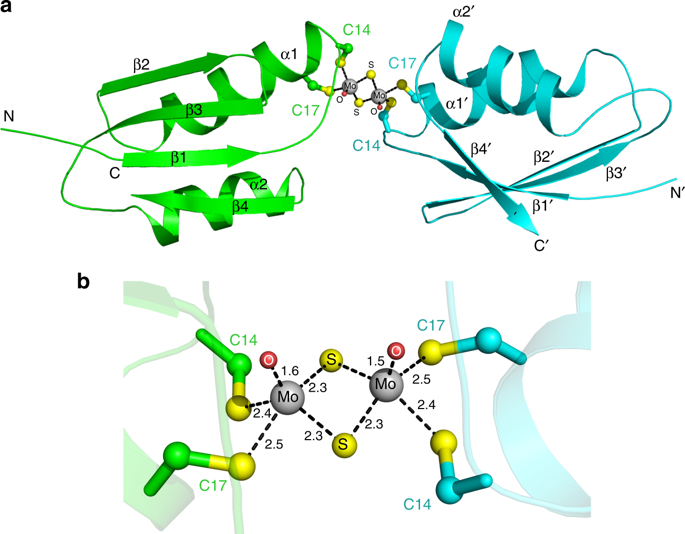
Tetrathiomolybdate (TM) is used in the clinic for the treatment of Wilson's disease by targeting the cellular copper efflux protein ATP7B (WLN). Interestingly, both TM and WLN are associated with the efficacy of cisplatin, a widely used anticancer drug. Herein, we show that TM induces dimerization of the metal-binding domain of ATP7B (WLN4) through a unique sulfur-bridged Mo2S6O2 cluster. TM expels copper ions from Cu-WLN4 and forms a copper-free dimer. The binding of Mo to cysteine residues of WLN4 inhibits platination of the protein. Reaction with multi-domain proteins indicates that TM can also connect two domains in the same molecule, forming Mo-bridged intramolecular crosslinks. These results provide structural and chemical insight into the mechanism of action of TM against ATPase, and reveal the molecular mechanism by which TM attenuates the cisplatin resistance mediated by copper efflux proteins.
中文翻译:

四硫代钼酸盐诱导ATPase的金属结合结构域二聚化,并抑制蛋白质的平台化。
通过靶向细胞铜外排蛋白ATP7B(WLN),四硫代钼酸盐(TM)在临床上用于治疗威尔逊氏病。有趣的是,TM和WLN都与顺铂(一种广泛使用的抗癌药)的功效有关。在这里,我们显示TM通过独特的硫桥联Mo2S6O2簇诱导ATP7B(WLN4)的金属结合域的二聚化。TM将铜离子从Cu-WLN4中排出,形成无铜二聚体。Mo与WLN4的半胱氨酸残基结合可抑制蛋白质的平台化。与多结构域蛋白的反应表明,TM也可以连接同一分子中的两个结构域,从而形成Mo桥连的分子内交联。这些结果为TM对抗ATPase的作用机理提供了结构和化学方面的见解,
更新日期:2019-01-14
中文翻译:

四硫代钼酸盐诱导ATPase的金属结合结构域二聚化,并抑制蛋白质的平台化。
通过靶向细胞铜外排蛋白ATP7B(WLN),四硫代钼酸盐(TM)在临床上用于治疗威尔逊氏病。有趣的是,TM和WLN都与顺铂(一种广泛使用的抗癌药)的功效有关。在这里,我们显示TM通过独特的硫桥联Mo2S6O2簇诱导ATP7B(WLN4)的金属结合域的二聚化。TM将铜离子从Cu-WLN4中排出,形成无铜二聚体。Mo与WLN4的半胱氨酸残基结合可抑制蛋白质的平台化。与多结构域蛋白的反应表明,TM也可以连接同一分子中的两个结构域,从而形成Mo桥连的分子内交联。这些结果为TM对抗ATPase的作用机理提供了结构和化学方面的见解,

































 京公网安备 11010802027423号
京公网安备 11010802027423号