Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2019-01-11 , DOI: 10.1016/j.jfluchem.2018.12.008
Abdelilah Benallou , Zouhair Lakbaibi , Hocine Garmes , Habib El Alaoui El Abdallaoui
|
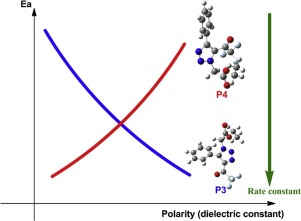
The goal of the present study is to make a comprehension of the connection between selectivity and polarity, in that fact this reaction process was studied in presence of toluene, THF and DMSO solvent through Molecular Electron Density Theory (MEDT) at the B3LYP/6-31+G(d,p) computational level. A remarkable predominance of 4-trifluoroacetyl-1,2,3-triazoles derivatives (P3) has been generated via [3 + 2] cycloaddition between CF3-ynone and azide ylide, rationalized by the electrophilic P+ and nucleophilic P− Parr functions at the reactive sites of reagents, affording the N3-C1 regioselectivity observed experimentally. Furthermore, an exploration of the relative energies of reaction, Gibbs free energy and barrier activation profile obviously evidences that experimentally isolated P3 is the most reachable product of this reaction, in good to excellent yield with respect to the increase of polarity. Moreover, ELF topology analysis of the most stable product demonstrates that the formation of new bonds appears to be directed through two dissimilar fashions, the first is formed by donation of some nitrogen non-bonding electron-density to the carbon pseudoradical center and, the second is formed by sharing nitrogen non-bonding electron-density and a carbon pseudoradical center via a non-concerted two-stage one-step molecular mechanism.
中文翻译:

极性对CF3-炔酮内酯与叠氮化物基团[3 + 2]环加成反应的机理和选择性的作用:量子化学研究
本研究的目的是要理解选择性和极性之间的联系,事实上,该反应过程是通过分子电子密度理论(MEDT)在B3LYP / 6-上在甲苯,THF和DMSO溶剂存在下进行研究的。 31 + G(d,p)的计算级别。的4-三氟乙酰基的1,2,3-三唑衍生物(P3)的一个显着优势已经经由[3 + 2] CF 3 - ynone和叠氮叶立德之间的环加成,通过亲电子合理化生成P +和亲核P -Parr在试剂的反应位点起作用,提供实验观察到的N3-C1区域选择性。此外,对反应的相对能量,吉布斯自由能和势垒活化曲线的探索显然证明,实验分离出的P3是该反应最可及的产物,相对于极性的增加,收率良好。此外,对最稳定的产物进行的ELF拓扑分析表明,新键的形成似乎是通过两种不同的方式进行的,第一种是通过向碳假自由基中心提供一些氮非键电子密度来形成的,第二种是通过非共构的两阶段单步分子机理,通过共享氮的非键电子密度和碳的伪自由基中心形成碳原子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号