当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The synthesis and characterization of giant Calixarenes.
Nature Communications ( IF 14.7 ) Pub Date : 2019-Jan-10 , DOI: 10.1038/s41467-018-07751-4 Vincent Guérineau , Marion Rollet , Stéphane Viel , Bénédicte Lepoittevin , Ludovic Costa , Pascale Saint-Aguet , Régis Laurent , Philippe Roger , Didier Gigmes , Cyril Martini , Vincent Huc
Nature Communications ( IF 14.7 ) Pub Date : 2019-Jan-10 , DOI: 10.1038/s41467-018-07751-4 Vincent Guérineau , Marion Rollet , Stéphane Viel , Bénédicte Lepoittevin , Ludovic Costa , Pascale Saint-Aguet , Régis Laurent , Philippe Roger , Didier Gigmes , Cyril Martini , Vincent Huc
|
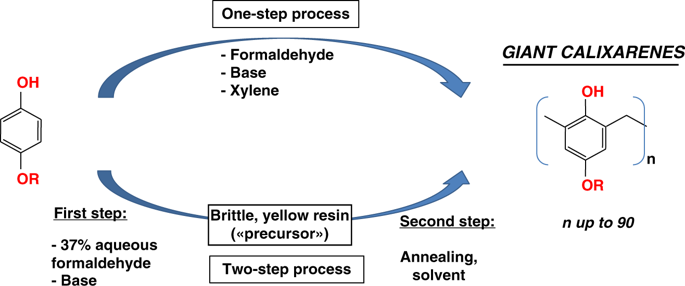
Calixarenes are cyclic oligomers obtained by condensation of suitable p-functionalised phenols with formaldehyde, usually allowing for the synthesis of the well known small calixarenes (including up to eight phenolic subunits). We report here the discovery of much larger members of this family, exhibiting sizes up to 90 phenolic subunits: the giant calixarenes. These macrocycles are obtained according to simple, easily scalable processes, in yields up to 65%. We show that the formation of these giant macrocycles is favored by an oxygen-containing-group at the para-position of the starting phenol, high concentrations of heavy alkaline bases (rubidium or cesium hydroxides) and long reaction times. A mechanism is proposed to rationalize these observations. These giant macrocycles can also be obtained in the quasi-solid state, opening interesting perspectives in the field of calixarenes chemistry. Along with their intrinsic fundamental interest, these objects are also opening interesting applicative potentialities.
中文翻译:

大杯芳烃的合成与表征。
杯芳烃是通过合适的对-官能化的苯酚与甲醛缩合而获得的环状低聚物,通常允许合成众所周知的小杯芳烃(包括多达八个酚亚基)。我们在这里报告了这个家族更大成员的发现,它们的大小高达90个酚亚基:巨大的杯芳烃。这些大环是通过简单,易于扩展的过程获得的,产率高达65%。我们表明,这些巨大的大环的形成受到起始苯酚对位的含氧基团,高浓度的重碱性碱(rub或氢氧化铯)和长反应时间的青睐。提出了一种使这些观察合理化的机制。这些巨大的大环也可以在准固态下获得,在杯芳烃化学领域开辟了有趣的观点。除了其内在的基本兴趣,这些对象还打开了有趣的应用潜力。
更新日期:2019-01-10
中文翻译:

大杯芳烃的合成与表征。
杯芳烃是通过合适的对-官能化的苯酚与甲醛缩合而获得的环状低聚物,通常允许合成众所周知的小杯芳烃(包括多达八个酚亚基)。我们在这里报告了这个家族更大成员的发现,它们的大小高达90个酚亚基:巨大的杯芳烃。这些大环是通过简单,易于扩展的过程获得的,产率高达65%。我们表明,这些巨大的大环的形成受到起始苯酚对位的含氧基团,高浓度的重碱性碱(rub或氢氧化铯)和长反应时间的青睐。提出了一种使这些观察合理化的机制。这些巨大的大环也可以在准固态下获得,在杯芳烃化学领域开辟了有趣的观点。除了其内在的基本兴趣,这些对象还打开了有趣的应用潜力。













































 京公网安备 11010802027423号
京公网安备 11010802027423号