当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Selective Lysine Reactions Guided by Protein–Peptide Interaction
Biochemistry ( IF 2.9 ) Pub Date : 2019-01-09 00:00:00 , DOI: 10.1021/acs.biochem.8b01223
Yu Zhang 1 , Yujie Liang 1 , Feng Huang 1 , Yue Zhang 2 , Xuechen Li 2 , Jiang Xia 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-01-09 00:00:00 , DOI: 10.1021/acs.biochem.8b01223
Yu Zhang 1 , Yujie Liang 1 , Feng Huang 1 , Yue Zhang 2 , Xuechen Li 2 , Jiang Xia 1
Affiliation
|
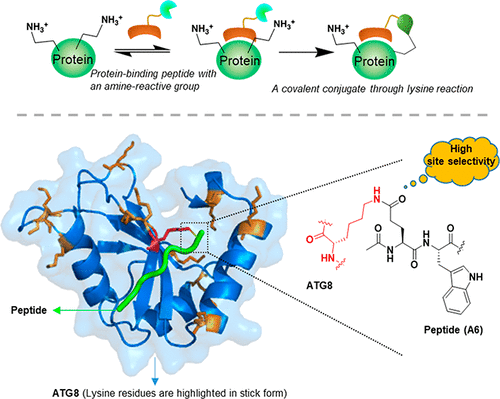
Site-selective lysine post-translational modifications such as acetylation, methylation, hydroxylation, and isopeptide formation mediate the precise control of important signaling events in cells with unmistakable accuracy. This unparalleled site selectivity (modification of a single lysine in a particular protein in the proteome) is still a challenge for non-enzymatic protein reactions; the difficulty lies in the differentiation of the lysine ε-amino group from other reactive groups and in the precise pinpointing of one particular lysine ε-amino group out of many other lysine ε-amino groups and the N-terminal amine of the protein that have similar chemical reactivity. Here, we have explored proximal lysine conjugation reactions through peptide-guided fluorodinitrobenzene, isothiocyanate, and phenyl ester reactions and have validated the site-specific targeting of the ε-amino group of one single lysine in natural proteins that contain multiple lysine residues. This precise site selectivity is a result of the proximity-induced reactivity guided by a specific protein–peptide interaction: the binding interaction preorganizes an amine-reactive group in the peptide and one of the lysine side chain ε-amino groups of the protein into close proximity, thereby confining the reactivity to a selected area of the target protein. The binding-guide lysine reactions were first examined on an SH3 domain and then tested on several ubiquitin-like proteins such as SUMO, Atg8 protein family, plant ATG8, and mammalian LC3 proteins that contain at least seven lysine residues on the surface. Exquisite site selectivity was confirmed in all of the proteins tested. A set of amine reactions were tested for their feasibility in the site-selective lysine reaction. Selected amine-reactive groups were optimized, and the reaction sites on the LC3 protein were confirmed by mass spectrometry.
中文翻译:

蛋白质-肽相互作用指导的位点选择性赖氨酸反应
位点选择性赖氨酸翻译后修饰,例如乙酰化,甲基化,羟基化和异肽形成,以毫无疑问的准确性介导了细胞中重要信号事件的精确控制。这种无与伦比的位点选择性(蛋白质组中特定蛋白质中单个赖氨酸的修饰)仍然是非酶促蛋白质反应的挑战。困难在于赖氨酸ε-氨基与其他反应基团的区别,以及从许多其他赖氨酸ε-氨基和蛋白质的N末端胺中精确地确定一个特定的赖氨酸ε-氨基。化学反应性相似。在这里,我们通过肽引导的氟二硝基苯,异硫氰酸酯探索了近端赖氨酸结合反应,和苯酯反应,并验证了含有多个赖氨酸残基的天然蛋白质中单个赖氨酸的ε-氨基的定点靶向。这种精确的位点选择性是特定蛋白质与肽相互作用指导的邻近诱导反应性的结果:结合相互作用使肽中的胺反应基团和蛋白质的赖氨酸侧链ε-氨基之一紧密结合在一起邻近,从而将反应性限制在靶蛋白的选定区域。首先在SH3结构域上检查结合引导的赖氨酸反应,然后在表面上包含至少七个赖氨酸残基的几种泛素样蛋白(例如SUMO,Atg8蛋白家族,植物ATG8和哺乳动物LC3蛋白)上进行测试。在所有测试的蛋白质中均证实了出色的位点选择性。测试了一组胺反应在定点赖氨酸反应中的可行性。优化选择的胺反应性基团,并通过质谱确定LC3蛋白上的反应位点。
更新日期:2019-01-09
中文翻译:

蛋白质-肽相互作用指导的位点选择性赖氨酸反应
位点选择性赖氨酸翻译后修饰,例如乙酰化,甲基化,羟基化和异肽形成,以毫无疑问的准确性介导了细胞中重要信号事件的精确控制。这种无与伦比的位点选择性(蛋白质组中特定蛋白质中单个赖氨酸的修饰)仍然是非酶促蛋白质反应的挑战。困难在于赖氨酸ε-氨基与其他反应基团的区别,以及从许多其他赖氨酸ε-氨基和蛋白质的N末端胺中精确地确定一个特定的赖氨酸ε-氨基。化学反应性相似。在这里,我们通过肽引导的氟二硝基苯,异硫氰酸酯探索了近端赖氨酸结合反应,和苯酯反应,并验证了含有多个赖氨酸残基的天然蛋白质中单个赖氨酸的ε-氨基的定点靶向。这种精确的位点选择性是特定蛋白质与肽相互作用指导的邻近诱导反应性的结果:结合相互作用使肽中的胺反应基团和蛋白质的赖氨酸侧链ε-氨基之一紧密结合在一起邻近,从而将反应性限制在靶蛋白的选定区域。首先在SH3结构域上检查结合引导的赖氨酸反应,然后在表面上包含至少七个赖氨酸残基的几种泛素样蛋白(例如SUMO,Atg8蛋白家族,植物ATG8和哺乳动物LC3蛋白)上进行测试。在所有测试的蛋白质中均证实了出色的位点选择性。测试了一组胺反应在定点赖氨酸反应中的可行性。优化选择的胺反应性基团,并通过质谱确定LC3蛋白上的反应位点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号