当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using the Analytical Target Profile to Drive the Analytical Method Lifecycle.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-01-09 00:00:00 , DOI: 10.1021/acs.analchem.8b04596 Patrick Jackson 1 , Phil Borman 1 , Cristiana Campa 2 , Marion Chatfield 1 , Mark Godfrey 3 , Peter Hamilton 1 , Walter Hoyer 4 , Francesco Norelli 2 , Rachel Orr 1 , Tim Schofield 5
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-01-09 00:00:00 , DOI: 10.1021/acs.analchem.8b04596 Patrick Jackson 1 , Phil Borman 1 , Cristiana Campa 2 , Marion Chatfield 1 , Mark Godfrey 3 , Peter Hamilton 1 , Walter Hoyer 4 , Francesco Norelli 2 , Rachel Orr 1 , Tim Schofield 5
Affiliation
|
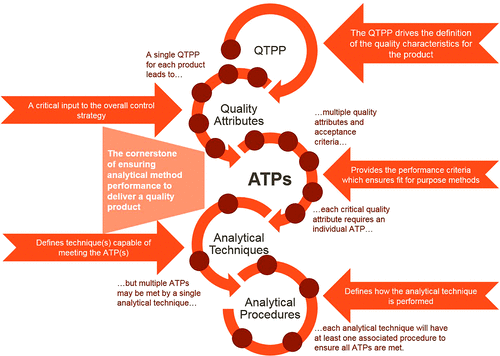
Quality by design (ICH-Topic Q8) requires a prospective summary of the desired quality characteristics of a drug product. This is known as the Quality Target Product Profile (QTPP), which forms the basis for the design and development of the product. An analogous term has been established for analytical procedures called the Analytical Target Profile (ATP). The ATP, in a similar fashion to the QTPP, prospectively summarizes the requirements associated with a measurement on a quality attribute which needs to be met by an analytical procedure. Criteria defined in the ATP relate to the maximum uncertainty associated with the reportable result that is required to maintain acceptable confidence in the quality decision made from the result. The ATP is used to define and assess the fitness of an analytical procedure in the development phase and during all changes across the analytical lifecycle. One or more analytical procedures can meet the requirements of an ATP. The ATP can be applied to any quality attribute across any pharmaceutical modality where an analytical procedure is used to generate a reportable result, and this paper provides examples from three of these modalities: small molecules, oligonucleotides, and vaccines. Some key performance characteristics will be discussed for each ATP, namely specificity, accuracy, and precision, taking into account the expected range of the analyte. The combination of accuracy and precision into a combined uncertainty characteristic is also discussed as a more holistic approach. The use of the ATP concept will help focus attention on the properties of a method which impact quality decisions rather than method descriptions and may enable greater regulatory flexibility across the lifecycle using established conditions based on method performance criteria as proposed in the Step 2 version of ICHQ12. The revision of ICHQ2(R1) and development of the new ICHQ14 guideline (Analytical Procedure Development) will provide a golden opportunity to harmonize the definition of new QbD concepts such as the ATP.
中文翻译:

使用分析目标配置文件来驱动分析方法生命周期。
设计质量(ICH-Topic Q8)需要对药品的所需质量特性进行前瞻性总结。这就是所谓的质量目标产品资料(QTPP),它构成了产品设计和开发的基础。对于分析程序,已经建立了一个类似的术语,称为“分析目标配置文件”(ATP)。ATP以与QTPP相似的方式前瞻性地总结了与质量属性测量相关的要求,这些要求需要通过分析程序来满足。ATP中定义的标准与可报告结果相关的最大不确定性有关,该不确定性要求对结果做出的质量决策保持可接受的置信度。ATP用于定义和评估分析程序在开发阶段以及整个分析生命周期中所有更改期间的适用性。一种或多种分析程序可以满足ATP的要求。ATP可以应用于任何药物模式中的任何质量属性,其中使用分析程序来生成可报告的结果,本文提供了以下三种模式的示例:小分子,寡核苷酸和疫苗。考虑到分析物的预期范围,将讨论每种ATP的一些关键性能特征,即特异性,准确性和精密度。作为更全面的方法,还讨论了将精度和精度组合为组合的不确定性特征。ATP概念的使用将有助于将注意力集中在影响质量决策的方法属性上,而不是方法描述上,并且可以使用基于方法性能标准的已建立条件(如ICHQ12第2版中建议的那样)在整个生命周期中实现更大的监管灵活性。 。ICHQ2(R1)的修订版和新的ICHQ14指南(分析程序开发)的开发将为统一新的QbD概念(如ATP)的定义提供一个千载难逢的机会。
更新日期:2019-01-09
中文翻译:

使用分析目标配置文件来驱动分析方法生命周期。
设计质量(ICH-Topic Q8)需要对药品的所需质量特性进行前瞻性总结。这就是所谓的质量目标产品资料(QTPP),它构成了产品设计和开发的基础。对于分析程序,已经建立了一个类似的术语,称为“分析目标配置文件”(ATP)。ATP以与QTPP相似的方式前瞻性地总结了与质量属性测量相关的要求,这些要求需要通过分析程序来满足。ATP中定义的标准与可报告结果相关的最大不确定性有关,该不确定性要求对结果做出的质量决策保持可接受的置信度。ATP用于定义和评估分析程序在开发阶段以及整个分析生命周期中所有更改期间的适用性。一种或多种分析程序可以满足ATP的要求。ATP可以应用于任何药物模式中的任何质量属性,其中使用分析程序来生成可报告的结果,本文提供了以下三种模式的示例:小分子,寡核苷酸和疫苗。考虑到分析物的预期范围,将讨论每种ATP的一些关键性能特征,即特异性,准确性和精密度。作为更全面的方法,还讨论了将精度和精度组合为组合的不确定性特征。ATP概念的使用将有助于将注意力集中在影响质量决策的方法属性上,而不是方法描述上,并且可以使用基于方法性能标准的已建立条件(如ICHQ12第2版中建议的那样)在整个生命周期中实现更大的监管灵活性。 。ICHQ2(R1)的修订版和新的ICHQ14指南(分析程序开发)的开发将为统一新的QbD概念(如ATP)的定义提供一个千载难逢的机会。






























 京公网安备 11010802027423号
京公网安备 11010802027423号