当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regiospecifically Fluorinated Polycyclic Aromatic Hydrocarbons via Julia–Kocienski Olefination and Oxidative Photocyclization. Effect of Fluorine Atom Substitution on Molecular Shape
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-03-22 00:00:00 , DOI: 10.1021/acs.joc.5b02580 Shaibal Banerjee 1 , Saikat Sinha 1 , Padmanava Pradhan 1 , Alessio Caruso 2 , Daniel Liebowitz 2 , Damon Parrish 3 , Miriam Rossi 2 , Barbara Zajc 1, 4
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-03-22 00:00:00 , DOI: 10.1021/acs.joc.5b02580 Shaibal Banerjee 1 , Saikat Sinha 1 , Padmanava Pradhan 1 , Alessio Caruso 2 , Daniel Liebowitz 2 , Damon Parrish 3 , Miriam Rossi 2 , Barbara Zajc 1, 4
Affiliation
|
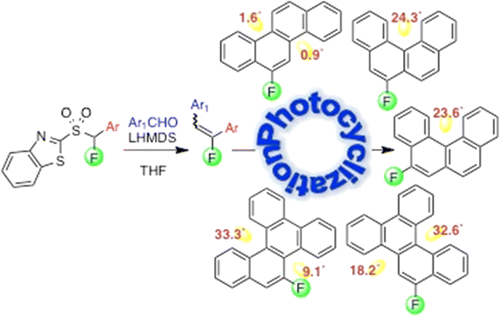
A modular synthesis of regiospecifically fluorinated polycyclic aromatic hydrocarbons (PAHs) is described. 1,2-Diarylfluoroalkenes, synthesized via Julia–Kocienski olefination (70–99% yields), were converted to isomeric 5- and 6-fluorobenzo[c]phenanthrene, 5-and 6-fluorochrysene, and 9- and 10-benzo[g]chrysene (66–83% yields) by oxidative photocyclization. Photocyclization to 6-fluorochrysene proceeded more slowly than conversion of 1-styrylnaphthalene to chrysene. Higher fluoroalkene dilution led to a more rapid cyclization. Therefore, photocyclizations were performed at higher dilutions. To evaluate the effect of fluorine atom on molecular shapes, X-ray data for 5- and 6-fluorobenzo[c]phenanthrene, 6-fluorochrysene, 9- and 10-fluorobenzo[g]chrysene, and unfluorinated chrysene as well as benzo[g]chrysene were obtained and compared. The fluorine atom caused a small deviation from planarity in the chrysene series and decreased nonplanarity in the benzo[c]phenanthrene derivatives, but its influence was most pronounced in the benzo[g]chrysene series. A remarkable flattening of the molecule was observed in 9-fluorobenzo[g]chrysene, where the short 2.055 Å interatomic distance between bay-region F-9 and H-8, downfield shift of H-8, and a 26.1 Hz coupling between F-9 and C-8 indicate a possible F-9···H-8 hydrogen bond. In addition, in 9-fluorobenzo[g]chrysene, the stacking distance is short at 3.365 Å and there is an additional interaction between the C-11–H and C-10a of a nearby molecule that is almost perpendicular.
中文翻译:

通过 Julia-Kocienski 烯化和氧化光环化制备区域特异性氟化多环芳烃。氟原子取代对分子形状的影响
描述了区域特异性氟化多环芳烃 (PAH) 的模块化合成。通过 Julia-Kocienski 烯化合成的 1,2-二芳基氟烯烃(产率 70-99%)被转化为异构体 5- 和 6-氟苯并[ c ]菲、5-和 6-氟苯以及 9- 和 10-苯并[ g ]chrysene(66-83%产率)通过氧化光环化。光环化为 6-氟 比 1-苯乙烯基萘 转化为 进行得更慢。氟代烯烃稀释度越高,环化速度越快。因此,光环化在更高的稀释度下进行。为了评估氟原子对分子形状的影响,使用了 5-和 6-氟苯并[ c ]菲、6-氟苯并[ g ]、未氟化以及苯并[g]的 X 射线数据。 g ]chrysene 得到并比较。氟原子导致了系列中平面性的微小偏差,并降低了苯并[ c ]菲衍生物中的非平面性,但其影响在苯并[ g ]系列中最为明显。在 9-氟苯并[ g ]chrysene 中观察到分子的显着扁平化,其中湾区 F-9 和 H-8 之间的短 2.055 Å 原子间距离、H-8 的低场位移以及 F 之间的 26.1 Hz 耦合-9和C-8表示可能的F-9…H-8氢键。此外,在 9-氟苯并[ g ]chrysene 中,堆积距离很短,为 3.365 Å,并且附近分子的 C-11–H 和 C-10a 之间几乎垂直,存在额外的相互作用。
更新日期:2016-03-22
中文翻译:

通过 Julia-Kocienski 烯化和氧化光环化制备区域特异性氟化多环芳烃。氟原子取代对分子形状的影响
描述了区域特异性氟化多环芳烃 (PAH) 的模块化合成。通过 Julia-Kocienski 烯化合成的 1,2-二芳基氟烯烃(产率 70-99%)被转化为异构体 5- 和 6-氟苯并[ c ]菲、5-和 6-氟苯以及 9- 和 10-苯并[ g ]chrysene(66-83%产率)通过氧化光环化。光环化为 6-氟 比 1-苯乙烯基萘 转化为 进行得更慢。氟代烯烃稀释度越高,环化速度越快。因此,光环化在更高的稀释度下进行。为了评估氟原子对分子形状的影响,使用了 5-和 6-氟苯并[ c ]菲、6-氟苯并[ g ]、未氟化以及苯并[g]的 X 射线数据。 g ]chrysene 得到并比较。氟原子导致了系列中平面性的微小偏差,并降低了苯并[ c ]菲衍生物中的非平面性,但其影响在苯并[ g ]系列中最为明显。在 9-氟苯并[ g ]chrysene 中观察到分子的显着扁平化,其中湾区 F-9 和 H-8 之间的短 2.055 Å 原子间距离、H-8 的低场位移以及 F 之间的 26.1 Hz 耦合-9和C-8表示可能的F-9…H-8氢键。此外,在 9-氟苯并[ g ]chrysene 中,堆积距离很短,为 3.365 Å,并且附近分子的 C-11–H 和 C-10a 之间几乎垂直,存在额外的相互作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号