当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong Duschinsky Mixing Induced Breakdown of Kasha’s Rule in an Organic Phosphor
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acs.jpclett.8b03624 Lopa Paul 1 , Torsha Moitra 2 , Kenneth Ruud 3 , Swapan Chakrabarti 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acs.jpclett.8b03624 Lopa Paul 1 , Torsha Moitra 2 , Kenneth Ruud 3 , Swapan Chakrabarti 1
Affiliation
|
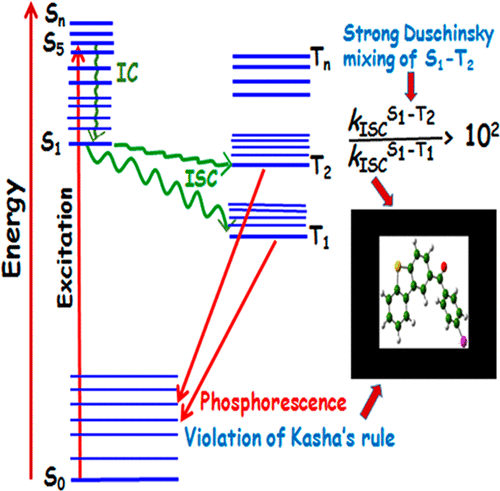
We present the novel observation that Duschinsky mixings can lead to the breakdown of Kasha’s rule in a white light phosphor molecule, dibenzo[b,d]thiophen-2-yl (4-chlorophenyl)methanone. Our theoretical analyses show the energy gap between the T1 and T2 states (0.48 eV) is too large to allow for any significant population of the T2 state at room temperature and instead the faster intersystem crossing (ISC) between the S1 and T2 states is rather due to strong Duschinsky mixing, leading to the emission from the T2 state as well. A second-order cumulant-based method has been used for the calculation of the ISC rate, which suggests 2 orders of magnitude faster ISC rates for S1 → T2 compared to those for S1 → T1. We found that the carbonyl moiety of the S1 and T2 states of the molecule is significantly different with respect to bond angle and dihedral angles, engendering large displacements in selective normal modes, thus giving rise to strong Duschinsky mixing.
中文翻译:

强烈的Duschinsky混合导致有机荧光体中Kasha规则的分解
我们提出了一种新颖的观察结果,即Duschinsky混合可导致白色荧光粉分子二苯并[ b,d ]噻吩-2-基(4-氯苯基)甲酮中的Kasha规则分解。我们的理论分析表明,T 1和T 2状态之间的能隙(0.48 eV)太大,无法在室温下容纳任何大量的T 2状态,而是S 1和T 2之间的更快的系统间穿越(ISC)T 2状态是由于强烈的Duschinsky混合导致的,导致T 2的发射状态。已使用基于二阶累积量的方法来计算ISC速率,这表明S 1 →T 2的ISC速率比S 1 →T 1的ISC速率快两个数量级。我们发现,分子的S 1和T 2状态的羰基部分在键角和二面角上存在显着差异,在选择性法线模式下产生大位移,从而引起强烈的Duschinsky混合。
更新日期:2019-01-08
中文翻译:

强烈的Duschinsky混合导致有机荧光体中Kasha规则的分解
我们提出了一种新颖的观察结果,即Duschinsky混合可导致白色荧光粉分子二苯并[ b,d ]噻吩-2-基(4-氯苯基)甲酮中的Kasha规则分解。我们的理论分析表明,T 1和T 2状态之间的能隙(0.48 eV)太大,无法在室温下容纳任何大量的T 2状态,而是S 1和T 2之间的更快的系统间穿越(ISC)T 2状态是由于强烈的Duschinsky混合导致的,导致T 2的发射状态。已使用基于二阶累积量的方法来计算ISC速率,这表明S 1 →T 2的ISC速率比S 1 →T 1的ISC速率快两个数量级。我们发现,分子的S 1和T 2状态的羰基部分在键角和二面角上存在显着差异,在选择性法线模式下产生大位移,从而引起强烈的Duschinsky混合。






























 京公网安备 11010802027423号
京公网安备 11010802027423号