当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Activation Effect of H2 Pretreatment on Ag/Al2O3 Catalyst for the Selective Oxidation of Ammonia
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-01-07 00:00:00 , DOI: 10.1021/acscatal.8b03744 Fei Wang 1, 2 , Guangzhi He 1 , Bo Zhang 1 , Min Chen 1 , Xueyan Chen 1, 2 , Changbin Zhang 1, 3 , Hong He 1, 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-01-07 00:00:00 , DOI: 10.1021/acscatal.8b03744 Fei Wang 1, 2 , Guangzhi He 1 , Bo Zhang 1 , Min Chen 1 , Xueyan Chen 1, 2 , Changbin Zhang 1, 3 , Hong He 1, 2, 3
Affiliation
|
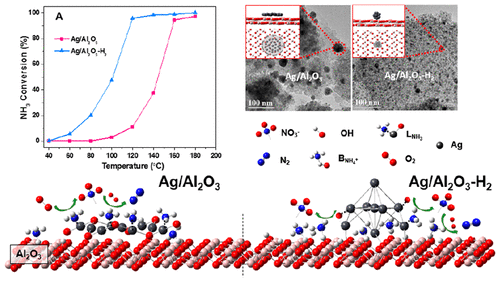
The Ag/Al2O3 catalyst possesses high activity for selective catalytic oxidation of ammonia (NH3–SCO), and its activity could be significantly improved by H2 pretreatment. It is generally accepted that the enhancement of Ag/Al2O3 activity after H2 reduction is due to the promoted O2 activation by metallic Ag species. Here, we show that the recovery of Brønsted acid sites via H2 reduction is also an important factor. Ag/Al2O3 and H2-pretreated Ag/Al2O3 (Ag/Al2O3–H2) catalysts were prepared and tested for the NH3–SCO performances, and also carefully characterized. It is revealed that Ag species were anchored on the Al2O3 surface through exchange between Ag+ and H+ in AlOH groups during the preparation process, and therefore occupied the Brønsted acid sites of Al2O3. H2 reduction could break the Ag–O bonds and thus recover the Brønsted acid sites. In situ DRIFTS results show that NH4+ species adsorbed on Brønsted acid sites is much more active for reaction with O2 than NH3 species adsorbed on Lewis acid sites. DFT calculation results identified the different activation processes for NH4+ and NH3 species, confirming that Brønsted acid sites have markedly higher activity than the Lewis acid sites. Therefore, the recovery of Brønsted acid sites after H2 reduction is also responsible for the improved activity of Ag/Al2O3–H2 for NH3–SCO.
中文翻译:

H 2预处理对Ag / Al 2 O 3氨选择性氧化催化剂活化作用的见解
Ag / Al 2 O 3催化剂对氨(NH 3 -SCO)的选择性催化氧化具有很高的活性,可通过H 2预处理显着提高其活性。普遍认为,H 2还原后,Ag / Al 2 O 3活性的增强是由于金属Ag物种促进了O 2的活化。在这里,我们表明通过H 2还原回收布朗斯台德酸位也是一个重要因素。Ag / Al 2 O 3和H 2预处理的Ag / Al 2 O 3(Ag / Al 2制备并测试了O 3 -H 2)催化剂的NH 3 -SCO性能,并进行了仔细表征。结果表明,在制备过程中,Ag通过AlOH基团中的Ag +与H +交换而锚固在Al 2 O 3表面,从而占据了Al 2 O 3的布朗斯台德酸位。H 2还原可能会破坏Ag-O键,从而恢复布朗斯台德酸位。原位DRIFTS结果表明,吸附在Brønsted酸位上的NH 4 +物种与O的反应活性更高。2比NH 3物种吸附在路易斯酸位点上。DFT计算结果确定了NH 4 +和NH 3物种的不同活化过程,从而证实了布朗斯台德酸位点比路易斯酸位点具有明显更高的活性。因此,H 2还原后布朗斯台德酸位的恢复也有助于提高Ag / Al 2 O 3 -H 2对NH 3 -SCO的活性。
更新日期:2019-01-07
中文翻译:

H 2预处理对Ag / Al 2 O 3氨选择性氧化催化剂活化作用的见解
Ag / Al 2 O 3催化剂对氨(NH 3 -SCO)的选择性催化氧化具有很高的活性,可通过H 2预处理显着提高其活性。普遍认为,H 2还原后,Ag / Al 2 O 3活性的增强是由于金属Ag物种促进了O 2的活化。在这里,我们表明通过H 2还原回收布朗斯台德酸位也是一个重要因素。Ag / Al 2 O 3和H 2预处理的Ag / Al 2 O 3(Ag / Al 2制备并测试了O 3 -H 2)催化剂的NH 3 -SCO性能,并进行了仔细表征。结果表明,在制备过程中,Ag通过AlOH基团中的Ag +与H +交换而锚固在Al 2 O 3表面,从而占据了Al 2 O 3的布朗斯台德酸位。H 2还原可能会破坏Ag-O键,从而恢复布朗斯台德酸位。原位DRIFTS结果表明,吸附在Brønsted酸位上的NH 4 +物种与O的反应活性更高。2比NH 3物种吸附在路易斯酸位点上。DFT计算结果确定了NH 4 +和NH 3物种的不同活化过程,从而证实了布朗斯台德酸位点比路易斯酸位点具有明显更高的活性。因此,H 2还原后布朗斯台德酸位的恢复也有助于提高Ag / Al 2 O 3 -H 2对NH 3 -SCO的活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号