当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Partial Oxidation of Methanol on the Fe3O4(111) Surface Studied by Density Functional Theory
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-01-22 , DOI: 10.1021/acs.jpcc.8b10557 Xiaoke Li 1 , Joachim Paier 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-01-22 , DOI: 10.1021/acs.jpcc.8b10557 Xiaoke Li 1 , Joachim Paier 1
Affiliation
|
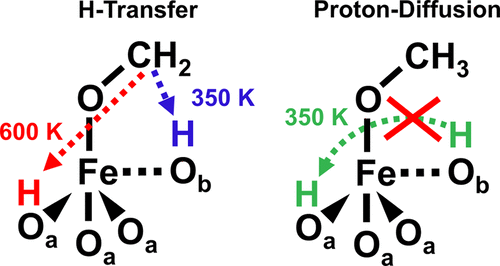
To understand recent temperature-programmed desorption (TPD) experiments carried out for methanol adsorbed on a Fe3O4(111) single crystal surface by Batista and co-workers, we accomplished a systematic density functional theory study on the various dehydrogenation pathways of methoxy species on that surface. For a mass/charge ratio of 30, these experiments detected two desorption peaks, one centered at about 330 and the second one at about 630 K, indicating that methoxide is partially oxidized to formaldehyde. Yet the origin of these two peaks has not been fully understood. Based on computed activation barriers for the H-transfer from methoxy species to the symmetrically distinct surface oxygen ions using the PBE+U approach and the HSE hybrid functional corrected for dispersion effects, we simulated the corresponding TPD peaks by numerical solution of a Polanyi–Wigner rate equation of first order. The simulated spectra using HSE suggest that the observed peaks are caused by the two inequivalent oxygen ions in the Fe3O4(111) surface. They have comparable activities in the protonation step upon adsorption of methanol but feature distinct reactivities toward the redox or H-transfer step causing significantly different activation barriers.
中文翻译:

密度泛函理论研究Fe 3 O 4(111)表面上甲醇的部分氧化
为了了解最近针对吸附在Fe 3 O 4上的甲醇进行的程序升温解吸(TPD)实验(111)由Batista及其同事制作的单晶表面,我们完成了关于该表面上甲氧基物质各种脱氢途径的系统密度泛函理论研究。对于30的质荷比,这些实验检测到两个解吸峰,一个解吸峰位于约330 K,第二个解吸峰位于约630 K,表明甲醇盐被部分氧化为甲醛。然而,这两个峰的起源还没有被完全理解。基于使用PBE + U方法计算出的从甲氧基物质到对称截然不同的表面氧离子的H转移的活化势垒和针对分散效应校正的HSE杂化功能,我们通过Polanyi–Wigner的数值解模拟了相应的TPD峰一阶速率方程。3 O 4(111)表面。它们在甲醇吸附的质子化步骤中具有可比的活性,但对氧化还原或H转移步骤具有明显的反应性,从而导致明显不同的活化势垒。
更新日期:2019-02-06
中文翻译:

密度泛函理论研究Fe 3 O 4(111)表面上甲醇的部分氧化
为了了解最近针对吸附在Fe 3 O 4上的甲醇进行的程序升温解吸(TPD)实验(111)由Batista及其同事制作的单晶表面,我们完成了关于该表面上甲氧基物质各种脱氢途径的系统密度泛函理论研究。对于30的质荷比,这些实验检测到两个解吸峰,一个解吸峰位于约330 K,第二个解吸峰位于约630 K,表明甲醇盐被部分氧化为甲醛。然而,这两个峰的起源还没有被完全理解。基于使用PBE + U方法计算出的从甲氧基物质到对称截然不同的表面氧离子的H转移的活化势垒和针对分散效应校正的HSE杂化功能,我们通过Polanyi–Wigner的数值解模拟了相应的TPD峰一阶速率方程。3 O 4(111)表面。它们在甲醇吸附的质子化步骤中具有可比的活性,但对氧化还原或H转移步骤具有明显的反应性,从而导致明显不同的活化势垒。






























 京公网安备 11010802027423号
京公网安备 11010802027423号