当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Remarkable Role of the Nitrogen Ligand in the Gas‐Phase Redox Reaction of the N2O/CO Couple Catalyzed by [NbN]+
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-02-04 , DOI: 10.1002/anie.201814460 Xiaoyan Sun 1 , Shaodong Zhou 1, 2 , Lei Yue 1 , Cheng Guo 1 , Maria Schlangen 1 , Helmut Schwarz 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-02-04 , DOI: 10.1002/anie.201814460 Xiaoyan Sun 1 , Shaodong Zhou 1, 2 , Lei Yue 1 , Cheng Guo 1 , Maria Schlangen 1 , Helmut Schwarz 1
Affiliation
|
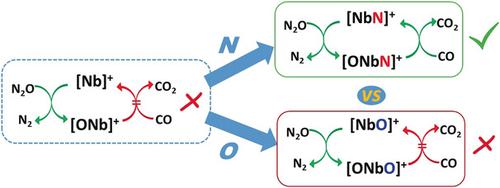
The thermal gas‐phase catalytic reduction of N2O by CO, mediated by the transition‐metal nitride cluster ion [NbN]+, has been explored by using FT‐ICR mass spectrometry and complemented by high‐level quantum chemical calculations. In contrast to the [Nb]+/[NbO]+ and [NbO]+/[Nb(O)2]+ systems, in which the oxidation of [Nb]+ and [NbO]+ with N2O is facile, but in which neither [NbO]+ nor [Nb(O)2]+ react with CO at room temperature, the [NbN]+/[ONbN]+ system at ambient temperature mediates the catalytic oxidation of CO. The origins of the distinctly different reactivities upon nitrogen ligation are addressed by quantum chemical calculations.
中文翻译:

氮配体在[NbN] +催化的N2O / CO对的气相氧化还原反应中的重要作用
过渡金属氮化物簇离子[NbN] +介导了CO对N 2 O的热气相催化还原,已通过FT-ICR质谱法进行了探索,并辅以高级量子化学计算。与[Nb] + / [NbO] +和[NbO] + / [Nb(O)2 ] +系统相反,其中[Nb] +和[NbO] +被N 2 O氧化很容易,但其中既不[NbO的] +也不[铌(O)2 ] +与CO反应在室温下,将[的NbN] + / [ONbN] + 系统在环境温度下介导CO的催化氧化。通过量子化学计算可解决氮连接后反应活性明显不同的起源。
更新日期:2019-02-04
中文翻译:

氮配体在[NbN] +催化的N2O / CO对的气相氧化还原反应中的重要作用
过渡金属氮化物簇离子[NbN] +介导了CO对N 2 O的热气相催化还原,已通过FT-ICR质谱法进行了探索,并辅以高级量子化学计算。与[Nb] + / [NbO] +和[NbO] + / [Nb(O)2 ] +系统相反,其中[Nb] +和[NbO] +被N 2 O氧化很容易,但其中既不[NbO的] +也不[铌(O)2 ] +与CO反应在室温下,将[的NbN] + / [ONbN] + 系统在环境温度下介导CO的催化氧化。通过量子化学计算可解决氮连接后反应活性明显不同的起源。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号