当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Octazethrene and Its Isomer with Different Diradical Characters and Chemical Reactivity: The Role of the Bridge Structure
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-03-21 00:00:00 , DOI: 10.1021/acs.joc.6b00172 Pan Hu 1 , Sangsu Lee 2 , Kyu Hyung Park 2 , Soumyajit Das 1 , Tun Seng Herng 3 , Théo P. Gonçalves 4 , Kuo-Wei Huang 4 , Jun Ding 3 , Dongho Kim 2 , Jishan Wu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-03-21 00:00:00 , DOI: 10.1021/acs.joc.6b00172 Pan Hu 1 , Sangsu Lee 2 , Kyu Hyung Park 2 , Soumyajit Das 1 , Tun Seng Herng 3 , Théo P. Gonçalves 4 , Kuo-Wei Huang 4 , Jun Ding 3 , Dongho Kim 2 , Jishan Wu 1
Affiliation
|
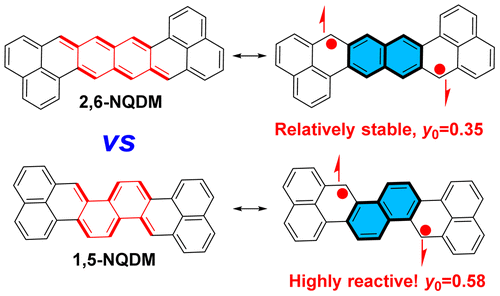
The fundamental relationship between structure and diradical character is important for the development of open-shell diradicaloid-based materials. In this work, we synthesized two structural isomers bearing a 2,6-naphthoquinodimethane or a 1,5-naphthoquinodimethane bridge and demonstrated that their diradical characters and chemical reactivity are quite different. The mesityl-or pentafluorophenyl-substituted octazethrene derivatives OZ-M/OZ-F and their isomer OZI-M (with mesityl substituents) were synthesized via an intramolecular Friedel–Crafts alkylation followed by oxidative dehydrogenation strategy from the key building blocks 4 and 11. Our detailed experimental and theoretical studies showed that both isomers have an open-shell singlet ground state with a remarkable diradical character (y0 = 0.35 and 0.34 for OZ-M and OZ-F, and y0 = 0.58 for OZI-M). Compounds OZ-M and OZ-F have good stability in an ambient environment, while OZI-M has high reactivity and can be easily oxidized to a dioxo product 15, which can be correlated to their different diradical characters. Additionally, we investigated the physical properties of OZ-M, OZ-F, and 15.
中文翻译:

具有不同双自由基特性和化学反应性的十八碳烯及其异构体:桥结构的作用
结构和双基特征之间的基本关系对于开壳双基基材料的开发很重要。在这项工作中,我们合成了带有2,6-萘醌二甲烷或1,5-萘醌二甲烷桥的两个结构异构体,并证明了它们的双自由基特性和化学反应性完全不同。由分子结构Friedel-Crafts烷基化,然后通过氧化脱氢策略,从关键结构单元4和11合成间苯三酚或五氟苯基取代的八氮杂蒽衍生物OZ-M / OZ-F及其异构体OZI-M(具有苯甲酰基取代基)。。我们的详细的实验和理论研究表明,两种异构体具有一个开壳层单重基态具有显着双基字符(Ý 0 = 0.35和0.34 OZ-M和OZ-F ,和ÿ 0 = 0.58 OZI-M )。化合物OZ-M和OZ-F在周围环境中具有良好的稳定性,而OZI-M具有高反应性并且可以容易地氧化成二氧羰基产物15,这可以与它们的不同的双自由基特性相关。此外,我们研究了OZ-M,OZ-F和15的物理性质。
更新日期:2016-03-21
中文翻译:

具有不同双自由基特性和化学反应性的十八碳烯及其异构体:桥结构的作用
结构和双基特征之间的基本关系对于开壳双基基材料的开发很重要。在这项工作中,我们合成了带有2,6-萘醌二甲烷或1,5-萘醌二甲烷桥的两个结构异构体,并证明了它们的双自由基特性和化学反应性完全不同。由分子结构Friedel-Crafts烷基化,然后通过氧化脱氢策略,从关键结构单元4和11合成间苯三酚或五氟苯基取代的八氮杂蒽衍生物OZ-M / OZ-F及其异构体OZI-M(具有苯甲酰基取代基)。。我们的详细的实验和理论研究表明,两种异构体具有一个开壳层单重基态具有显着双基字符(Ý 0 = 0.35和0.34 OZ-M和OZ-F ,和ÿ 0 = 0.58 OZI-M )。化合物OZ-M和OZ-F在周围环境中具有良好的稳定性,而OZI-M具有高反应性并且可以容易地氧化成二氧羰基产物15,这可以与它们的不同的双自由基特性相关。此外,我们研究了OZ-M,OZ-F和15的物理性质。































 京公网安备 11010802027423号
京公网安备 11010802027423号