当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling New Isomers and Rearrangement Routes on the C7H8+ Potential Energy Surface
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-01-04 00:00:00 , DOI: 10.1021/acs.jpca.8b10642 Ugo Jacovella 1 , Gabriel da Silva 2 , Evan J. Bieske 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-01-04 00:00:00 , DOI: 10.1021/acs.jpca.8b10642 Ugo Jacovella 1 , Gabriel da Silva 2 , Evan J. Bieske 1
Affiliation
|
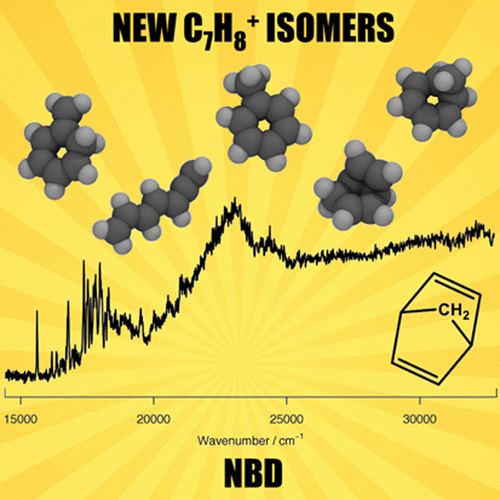
The unimolecular reactions of C7H8+ radical cations are among those most studied by mass spectrometry, especially the rearrangement of toluene and cycloheptatriene molecular ions, which are directly connected to the formation of benzylium and tropylium cations. This study reveals important new isomers and isomerization pathways on the C7H8+ potential energy surface, through the application of gas-phase electronic photodissociation spectroscopy in conjunction with ab initio calculations. Presented are the first gas-phase vibrationally resolved electronic spectra of the o-isotoluene, norcaradiene, bicyclo[3.2.0]hepta-2,6-diene radical cations, and ring-opened products from cyclic C7H8+ species. The isomerization route from the norbornadiene radical cation to the toluene radical cation, which competes with isomerization to the bicyclo[2.2.1]hepta-2-ene-5-yl-7-ylium radical cation, is identified. Further, this work expands understanding of the C7H8+ potential energy surface by connecting spiro[2.4]hepta-4,6-diene and acyclic 1,2,4,6-heptatetraene radical cations, and confirms the important role of the o-isotoluene radical cation in the interconversion pathways of C7H8+ species.
中文翻译:

在C 7 H 8 +势能面上揭示新的异构体和重排路线。
C 7 H 8 +自由基阳离子的单分子反应是质谱研究最深入的反应之一,尤其是甲苯和环庚三烯分子离子的重排,它们直接与苄基阳离子和对硝基阳离子的形成有关。这项研究揭示了重要的新异构体和C 7 H 8 +势能表面上的异构化途径,通过气相电子光解离光谱结合从头算的应用。呈现的是邻-异甲苯,正二十碳烯,双环[3.2.0]庚2,6-二烯自由基阳离子和环状C的开环产物的第一个气相振动分辨电子光谱7 H 8 +种。确定了从降冰片二烯自由基阳离子到甲苯自由基阳离子的异构化路线,该途径与异构化竞争为双环[2.2.1]庚-2--2-烯-5-基-7-自由基自由基阳离子。此外,这项工作通过连接螺[2.4]庚-4,6-二烯和无环1,2,4,6-庚四烯自由基阳离子扩展了对C 7 H 8 +势能面的认识,并确认了C 7 H 8 +势能表面的重要作用。C 7 H 8 +物种互变途径中的邻-异甲苯自由基阳离子。
更新日期:2019-01-04
中文翻译:

在C 7 H 8 +势能面上揭示新的异构体和重排路线。
C 7 H 8 +自由基阳离子的单分子反应是质谱研究最深入的反应之一,尤其是甲苯和环庚三烯分子离子的重排,它们直接与苄基阳离子和对硝基阳离子的形成有关。这项研究揭示了重要的新异构体和C 7 H 8 +势能表面上的异构化途径,通过气相电子光解离光谱结合从头算的应用。呈现的是邻-异甲苯,正二十碳烯,双环[3.2.0]庚2,6-二烯自由基阳离子和环状C的开环产物的第一个气相振动分辨电子光谱7 H 8 +种。确定了从降冰片二烯自由基阳离子到甲苯自由基阳离子的异构化路线,该途径与异构化竞争为双环[2.2.1]庚-2--2-烯-5-基-7-自由基自由基阳离子。此外,这项工作通过连接螺[2.4]庚-4,6-二烯和无环1,2,4,6-庚四烯自由基阳离子扩展了对C 7 H 8 +势能面的认识,并确认了C 7 H 8 +势能表面的重要作用。C 7 H 8 +物种互变途径中的邻-异甲苯自由基阳离子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号