当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Phenylpyridine- and 2-(benzo[b]thiophen-2-yl)pyridine-based o-carboranyl compounds: impact of the structural formation of aromatic rings on photophysical properties†
Dalton Transactions ( IF 3.5 ) Pub Date : 2019-01-03 00:00:00 , DOI: 10.1039/c8dt04367a Hyomin Jin 1, 2, 3, 4 , Hye Jin Bae 4, 5, 6, 7 , Seonah Kim 1, 2, 3, 4 , Ji Hye Lee 1, 2, 3, 4 , Hyonseok Hwang 1, 2, 3, 4 , Myung Hwan Park 4, 8, 9, 10 , Kang Mun Lee 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2019-01-03 00:00:00 , DOI: 10.1039/c8dt04367a Hyomin Jin 1, 2, 3, 4 , Hye Jin Bae 4, 5, 6, 7 , Seonah Kim 1, 2, 3, 4 , Ji Hye Lee 1, 2, 3, 4 , Hyonseok Hwang 1, 2, 3, 4 , Myung Hwan Park 4, 8, 9, 10 , Kang Mun Lee 1, 2, 3, 4
Affiliation
|
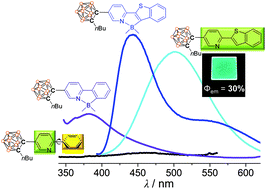
2-Phenylpyridine- and 2-(benzo[b]thiophen-2-yl)pyridine-based (ppy- and btp-based) o-carboranyl (Car1 and Car2) and their B(CH3)2-C∧N-chelated (Car1B and Car2B) compounds were prepared and fully characterised by multinuclear NMR spectroscopy and elemental analysis. The solid-state structure of Car2B was determined by single-crystal X-ray diffraction, which revealed a four-coordinated dimethylboryl centre. All compounds displayed major absorption bands that were assigned to π–π* transitions involving the ppy and btp moieties, as well as weak intramolecular charge-transfer (ICT) transitions between the o-carboranes and their aryl groups. Furthermore, the chelated compounds exhibited dominant low-energy absorption bands (λabs = 333 nm for Car1B and 383 nm for Car2B) resulting from the reinforcement of ICT transitions that correspond to the o-carborane moieties through the restriction of aromatic-ring free rotation. While Car1 and Car2 did not exhibit photoluminescence emissions in toluene at 298 K, Car1B and Car2B showed intense emissions, which are assignable to π–π* transitions associated with each chelated aryl group. However, Car1 and Car2 evidently emitted at around 450 nm in solution at 77 K, invoked by radiative ICT transitions between the carborane and the ppy or btp moiety, indicating that ICT-based radiative decay is only invigorated in the rigid state in the absence of structural variations, such as C–C bond fluctuations in the carborane cage and aromatic-ring free rotation. Interestingly, while Car1 in the film state exhibited a weak ICT-based emission spectrum, and Car1B and Car2B showed intense emissions originating from π–π* transitions associated with each chelated aryl group, Car2 showed significantly enhanced emissions in the same energy region as that exhibited in solution at 77 K, resulting in a much larger quantum efficiency over that in solution. DFT-optimised structures of Car1 and Car2 in their ground and the first-excited states clearly reveal that the enhanced emissive features of Car2 in the film state are strongly associated with the retained planarity of the btp moiety in both the ground and excited states. The photophysical results for these o-carboranyl compounds definitively reveal that the planarities of the aryl groups appended to the o-carborane decisively affect the efficiency of radiative decay based on ICT involving the o-carborane.
中文翻译:

基于2-苯基吡啶和2-(苯并[ b ]噻吩-2-基)吡啶的邻氨基甲酰基化合物:芳环结构形成对光物理性质的影响†
2- Phenylpyridine-和2-(苯并[ b ]噻吩-2-基)吡啶基(ppy-和BTP为主)Ö -carboranyl(分享帮助和CAR2)及其B(CH 3)2 - Ç ∧ ñ -制备了螯合的(Car1B和Car2B)化合物,并通过多核NMR光谱和元素分析对其进行了全面表征。Car2B的固态结构通过单晶X-射线衍射确定了X射线衍射,其揭示了四配位的二甲基硼基中心。所有化合物均显示出主要吸收带,这些吸收带被分配给涉及ppy和btp部分的π–π *跃迁,以及o-碳二烯与其芳基之间的弱分子内电荷转移(ICT)跃迁。此外,螯合化合物显示出占优势的低能量吸收带(λ ABS = 333纳米为Car1B和383纳米为Car2B)从ICT转变的加强得到对应于ö通过芳香环自由旋转的限制部分-carborane 。而Car1和Car2在298 K时甲苯中未显示出光致发光发射,Car1B和Car2B显示出强发射,这可归因于与每个螯合的芳基相关的π-π*跃迁。但是,Car1和Car2在77 K的溶液中明显在450 nm处发射,这是由碳硼烷与ppy或btp部分之间的辐射ICT跃迁引起的,表明基于ICT的辐射衰减仅在刚性状态下才得以增强,结构变化,例如碳硼烷笼中的C–C键波动和芳环自由旋转。有趣的是,虽然处于电影状态的Car1表现出较弱的基于ICT的发射光谱,而Car1B和Car2B由于显示出源自与每个螯合芳基相关的π–π *跃迁的强烈发射,Car2在与77K溶液中相同的能量区域中显示出明显增强的发射,从而导致量子效率大大高于溶液中的量子效率。DFT优化的Car1和Car2在其基态和第一激发态下的结构清楚地表明,在膜态下Car2增强的发射特征与btp基团在基态和激发态下保留的平面度密切相关。这些邻氨基甲酸酯基化合物的光物理结果明确地表明,连接到邻位的芳基的平面度碳杂烷基于涉及邻碳杂烷的ICT决定性地影响辐射衰减的效率。
更新日期:2019-01-03
中文翻译:

基于2-苯基吡啶和2-(苯并[ b ]噻吩-2-基)吡啶的邻氨基甲酰基化合物:芳环结构形成对光物理性质的影响†
2- Phenylpyridine-和2-(苯并[ b ]噻吩-2-基)吡啶基(ppy-和BTP为主)Ö -carboranyl(分享帮助和CAR2)及其B(CH 3)2 - Ç ∧ ñ -制备了螯合的(Car1B和Car2B)化合物,并通过多核NMR光谱和元素分析对其进行了全面表征。Car2B的固态结构通过单晶X-射线衍射确定了X射线衍射,其揭示了四配位的二甲基硼基中心。所有化合物均显示出主要吸收带,这些吸收带被分配给涉及ppy和btp部分的π–π *跃迁,以及o-碳二烯与其芳基之间的弱分子内电荷转移(ICT)跃迁。此外,螯合化合物显示出占优势的低能量吸收带(λ ABS = 333纳米为Car1B和383纳米为Car2B)从ICT转变的加强得到对应于ö通过芳香环自由旋转的限制部分-carborane 。而Car1和Car2在298 K时甲苯中未显示出光致发光发射,Car1B和Car2B显示出强发射,这可归因于与每个螯合的芳基相关的π-π*跃迁。但是,Car1和Car2在77 K的溶液中明显在450 nm处发射,这是由碳硼烷与ppy或btp部分之间的辐射ICT跃迁引起的,表明基于ICT的辐射衰减仅在刚性状态下才得以增强,结构变化,例如碳硼烷笼中的C–C键波动和芳环自由旋转。有趣的是,虽然处于电影状态的Car1表现出较弱的基于ICT的发射光谱,而Car1B和Car2B由于显示出源自与每个螯合芳基相关的π–π *跃迁的强烈发射,Car2在与77K溶液中相同的能量区域中显示出明显增强的发射,从而导致量子效率大大高于溶液中的量子效率。DFT优化的Car1和Car2在其基态和第一激发态下的结构清楚地表明,在膜态下Car2增强的发射特征与btp基团在基态和激发态下保留的平面度密切相关。这些邻氨基甲酸酯基化合物的光物理结果明确地表明,连接到邻位的芳基的平面度碳杂烷基于涉及邻碳杂烷的ICT决定性地影响辐射衰减的效率。































 京公网安备 11010802027423号
京公网安备 11010802027423号