当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Finding mechanochemical pathways and barriers without transition state search
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-05-05 15:03:16 , DOI: 10.1063/1.4919541 Stanislav M. Avdoshenko 1 , Dmitrii E. Makarov 1, 2
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-05-05 15:03:16 , DOI: 10.1063/1.4919541 Stanislav M. Avdoshenko 1 , Dmitrii E. Makarov 1, 2
Affiliation
|
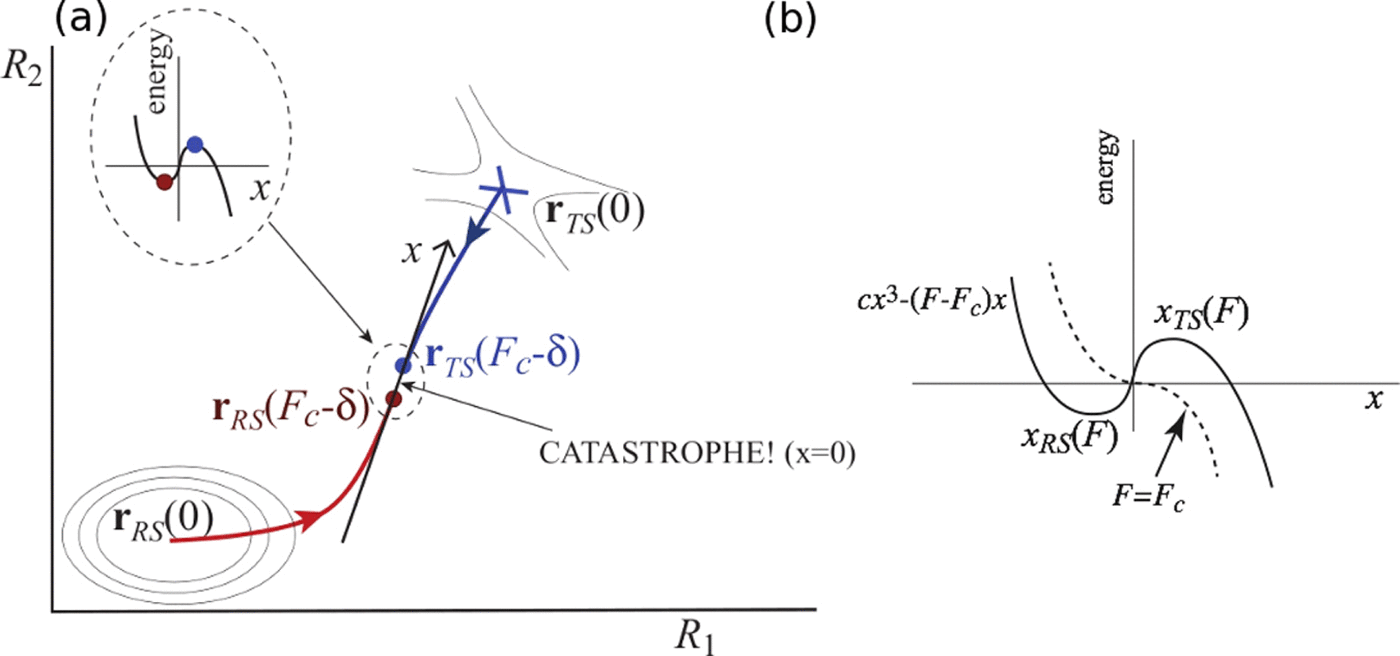
In covalent mechanochemistry, precise application of mechanical stress to molecules of interest (“mechanophores”) is used to induce to promote desired reaction pathways. Computational prediction of such phenomena and rational mechanophore design involves the computationally costly task of finding relevant transition-state saddles on force-deformed molecular potential energy surfaces (PESs). Finding a transition state often requires an initial guess about the pathway by which the reaction will proceed. Unfortunately, chemical intuition often fails when predicting likely consequences of mechanical stress applied to molecular systems. Here, we describe a fully deterministic method for finding mechanochemically relevant transition states and reaction pathways. The method is based on the observation that application of a sufficiently high mechanical force will eventually destabilize any molecular structure. Mathematically, such destabilization proceeds via a “catastrophe” occurring at a critical force where the energy minimum corresponding to the stable molecular structure coalesces with a transition state. Catastrophe theory predicts the force-deformed PES to have universal behavior in the vicinity of the critical force, allowing us to deduce the molecular structure of the transition state just below the critical force analytically. We then use the previously developed method of tracking transition-state evolution with the force to map out the entire reaction path and to predict the complete force dependence of the reaction barrier. Beyond its applications in mechanochemistry, this approach may be useful as a general method of finding transition states using fictitious forces to target specific reaction mechanisms.
中文翻译:

在没有过渡状态搜索的情况下寻找机械化学途径和障碍
在共价机械化学中,将机械应力精确施加到感兴趣的分子(“机械基团”)用于诱导促进所需的反应途径。这种现象的计算预测和合理的力学设计涉及在力变形的分子势能表面(PESs)上找到相关过渡态鞍的计算量大的任务。找到过渡状态通常需要对反应进行的途径进行初步猜测。不幸的是,当预测机械应力作用于分子系统的可能后果时,化学直觉通常会失败。在这里,我们描述了一种寻找机械化学相关的过渡态和反应途径的完全确定性的方法。该方法基于以下观察:施加足够高的机械力最终会破坏任何分子结构的稳定性。在数学上,这种不稳定作用是通过在临界力处发生的“灾难”而发生的,在临界力下,对应于稳定分子结构的最小能量与过渡态结合。突变理论预测,受力变形的PES在临界力附近具有普遍行为,这使我们能够通过分析推论出恰好低于临界力的过渡态的分子结构。然后,我们使用先前开发的通过力跟踪过渡态演化的方法来绘制整个反应路径并预测反应屏障的完全力依赖性。除了在机械化学中的应用外,
更新日期:2015-05-06
中文翻译:

在没有过渡状态搜索的情况下寻找机械化学途径和障碍
在共价机械化学中,将机械应力精确施加到感兴趣的分子(“机械基团”)用于诱导促进所需的反应途径。这种现象的计算预测和合理的力学设计涉及在力变形的分子势能表面(PESs)上找到相关过渡态鞍的计算量大的任务。找到过渡状态通常需要对反应进行的途径进行初步猜测。不幸的是,当预测机械应力作用于分子系统的可能后果时,化学直觉通常会失败。在这里,我们描述了一种寻找机械化学相关的过渡态和反应途径的完全确定性的方法。该方法基于以下观察:施加足够高的机械力最终会破坏任何分子结构的稳定性。在数学上,这种不稳定作用是通过在临界力处发生的“灾难”而发生的,在临界力下,对应于稳定分子结构的最小能量与过渡态结合。突变理论预测,受力变形的PES在临界力附近具有普遍行为,这使我们能够通过分析推论出恰好低于临界力的过渡态的分子结构。然后,我们使用先前开发的通过力跟踪过渡态演化的方法来绘制整个反应路径并预测反应屏障的完全力依赖性。除了在机械化学中的应用外,































 京公网安备 11010802027423号
京公网安备 11010802027423号