当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low Levels of Sox2 are required for Melanoma Tumor-Repopulating Cell Dormancy.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.29698 Qiong Jia 1 , Fang Yang 1 , Wei Huang 2 , Yao Zhang 1 , Binghao Bao 3 , Ke Li 1 , Fuxiang Wei 1 , Cunyu Zhang 1 , Haibo Jia 3
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.29698 Qiong Jia 1 , Fang Yang 1 , Wei Huang 2 , Yao Zhang 1 , Binghao Bao 3 , Ke Li 1 , Fuxiang Wei 1 , Cunyu Zhang 1 , Haibo Jia 3
Affiliation
|
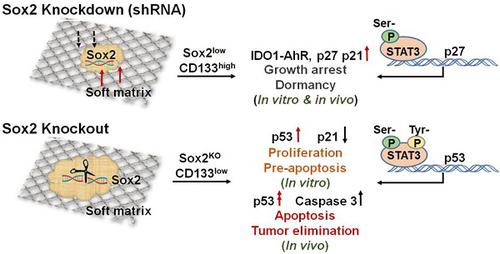
Tumorigenic cells, when facing a hostile environment, may enter a dormant state, leading to long-term tumor survival, relapse, and metastasis. To date, the molecular mechanism of tumor cell dormancy remains poorly understood. Methods: A soft, 3-dimentional (3D) fibrin gel culture system was used to mechanically select and grow highly malignant and tumorigenic melanoma tumor-repopulating cells (TRCs). We cultured control melanoma TRCs, TRCs with Sox2 knockdown, TRCs with Sox2 knockout, and a 2D control for in vitro and in vivo experiments. Western blotting, immunofluorescence, and flow cytometry analysis were performed to examine TRC dormancy and exit from dormancy. Results: Under a low-expression condition, we show that Sox2, a stemness molecule participates in dormancy regulation of highly tumorigenic cells that can repopulate a tumor (TRCs). Intriguingly, complete depletion of Sox2 via knockout results in dormancy exit and growth resumption of melanoma TRCs in culture and elevation of melanoma TRC apoptosis. Mice that are injected subcutaneously with Sox2-depleted melanoma TRCs do not form tumors and survive much longer than those injected with melanoma TRCs. We found that complete depletion of Sox2 promotes nuclear translocation of phosphorylated STAT3, where it binds to the p53 gene promoter, thus activating the p53-caspase3 cascade. Conclusion: These findings provide a novel insight into the role of the Sox2 gene in tumor cell stemness, tumor dormancy, and apoptosis.
中文翻译:

黑色素瘤肿瘤再生细胞休眠需要低水平的 Sox2。
致瘤细胞在面临恶劣环境时可能会进入休眠状态,导致肿瘤长期存活、复发和转移。迄今为止,人们对肿瘤细胞休眠的分子机制仍知之甚少。方法:使用柔软的三维 (3D) 纤维蛋白凝胶培养系统来机械选择和培养高度恶性和致瘤性黑色素瘤肿瘤再生细胞 (TRC)。我们培养了对照黑色素瘤 TRC、Sox2 敲低的 TRC、Sox2 敲除的 TRC 以及用于体外和体内实验的 2D 对照。进行蛋白质印迹、免疫荧光和流式细胞术分析来检查 TRC 休眠和退出休眠。结果:在低表达条件下,我们发现干性分子 Sox2 参与可重新增殖肿瘤 (TRC) 的高致瘤性细胞的休眠调节。有趣的是,通过敲除完全消除 Sox2 会导致培养物中黑色素瘤 TRC 休眠退出并恢复生长,并导致黑色素瘤 TRC 凋亡增加。皮下注射 Sox2 缺失的黑色素瘤 TRC 的小鼠不会形成肿瘤,并且比注射黑色素瘤 TRC 的小鼠存活时间更长。我们发现,Sox2 的完全耗尽会促进磷酸化 STAT3 的核转位,使其与 p53 基因启动子结合,从而激活 p53-caspase3 级联。结论:这些发现为了解 Sox2 基因在肿瘤细胞干性、肿瘤休眠和细胞凋亡中的作用提供了新的见解。
更新日期:2019-01-01
中文翻译:

黑色素瘤肿瘤再生细胞休眠需要低水平的 Sox2。
致瘤细胞在面临恶劣环境时可能会进入休眠状态,导致肿瘤长期存活、复发和转移。迄今为止,人们对肿瘤细胞休眠的分子机制仍知之甚少。方法:使用柔软的三维 (3D) 纤维蛋白凝胶培养系统来机械选择和培养高度恶性和致瘤性黑色素瘤肿瘤再生细胞 (TRC)。我们培养了对照黑色素瘤 TRC、Sox2 敲低的 TRC、Sox2 敲除的 TRC 以及用于体外和体内实验的 2D 对照。进行蛋白质印迹、免疫荧光和流式细胞术分析来检查 TRC 休眠和退出休眠。结果:在低表达条件下,我们发现干性分子 Sox2 参与可重新增殖肿瘤 (TRC) 的高致瘤性细胞的休眠调节。有趣的是,通过敲除完全消除 Sox2 会导致培养物中黑色素瘤 TRC 休眠退出并恢复生长,并导致黑色素瘤 TRC 凋亡增加。皮下注射 Sox2 缺失的黑色素瘤 TRC 的小鼠不会形成肿瘤,并且比注射黑色素瘤 TRC 的小鼠存活时间更长。我们发现,Sox2 的完全耗尽会促进磷酸化 STAT3 的核转位,使其与 p53 基因启动子结合,从而激活 p53-caspase3 级联。结论:这些发现为了解 Sox2 基因在肿瘤细胞干性、肿瘤休眠和细胞凋亡中的作用提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号