当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Atmospheres on the Initial Thermal Decomposition of 1,3,5-Trinitro-1,3,5-triazinane: Reactive Molecular Dynamics Simulation
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-01-07 , DOI: 10.1021/acs.jpcc.8b10360 Kai Zhong 1, 2 , Jian Liu 1 , Linyuan Wang 2 , Chaoyang Zhang 1, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-01-07 , DOI: 10.1021/acs.jpcc.8b10360 Kai Zhong 1, 2 , Jian Liu 1 , Linyuan Wang 2 , Chaoyang Zhang 1, 3
Affiliation
|
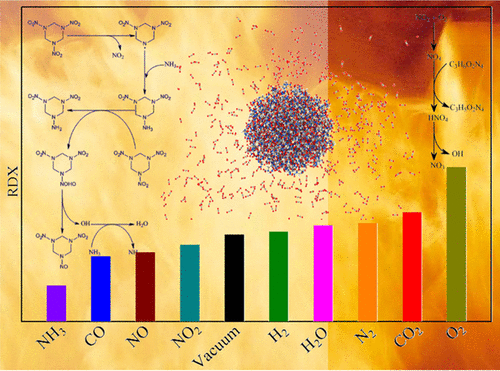
Energetic materials (EMs) are usually surrounded in atmospheres during manufacture, storage, transportation, and application. Thus, an insight into the influence of atmospheres on the EM decay becomes of significance. In the present work, molecular dynamics simulations are separately performed on 10 systems of one 1,3,5-trinitro-1,3,5-triazinane (RDX) nanoparticle in vacuum and in nine atmospheres including CO2, CO, H2O, H2, N2, NH3, O2, NO, and NO2 to identify the influence. No evident reaction between RDX and any atmosphere is found at room temperature, suggesting such a negligible influence. Nevertheless, the influences of the nine atmospheres are variable at high temperatures and can be classified into three cases. NH3, CO, NO, and NO2, in particular NH3, promote the RDX decay by consuming RDX and the intermediates generated from it. Because NO2 that serves as a catalyzer to accelerate the RDX decay is considerably consumed by O2, the decay is significantly prohibited by O2. In addition, a few prohibition effects of CO2, H2O, H2, and N2 on the decay are confirmed because of their dilution effects, with a few or even without reaction involving them. Besides, the NO2 partition dominates the initial steps of the RDX decay, followed by the ring cleavage. In addition, the population of ONDNTA that is an intermediate by partitioning one O atom from RDX is found to be indicative of the decay degree of RDX in various atmospheres. This work presents a comprehensive insight into the influence of atmospheres on the thermal decay of RDX.
中文翻译:

气氛对1,3,5-三硝基-1,3,5-三嗪烷初始热分解的影响:反应分子动力学模拟
在制造,存储,运输和应用过程中,高能材料(EMs)通常被包围在大气中。因此,深入了解大气对EM衰减的影响就变得很重要。在目前的工作中,分别在真空和包括CO 2,CO,H 2 O在内的九种气氛中,在10个1,3,5-三硝基-1,3,5-三嗪烷(RDX)纳米粒子的系统上进行分子动力学模拟,H 2,N 2,NH 3,O 2,NO和NO 2找出影响。在室温下,RDX与任何气氛之间均未发现明显反应,表明这种影响可忽略不计。然而,在高温下,这九种气氛的影响是可变的,可以分为三种情况。NH 3,CO,NO和NO 2,特别是NH 3,通过消耗RDX及其所产生的中间体来促进RDX的衰变。因为用作加速RDX衰变的催化剂的NO 2被O 2大量消耗,所以该衰变被O 2明显禁止。此外,CO 2,H 2 O,H 2和N的一些禁止作用由于它们的稀释作用,证实了对2的衰变,很少或什至没有涉及它们的反应。此外,NO 2分区主导了RDX衰变的初始步骤,随后发生环断裂。另外,发现通过将RDX中的一个O原子隔开而成为中间产物的ONDNTA的总体指示了RDX在各种气氛中的衰减程度。这项工作提供了对大气对RDX热衰减的影响的全面了解。
更新日期:2019-01-07
中文翻译:

气氛对1,3,5-三硝基-1,3,5-三嗪烷初始热分解的影响:反应分子动力学模拟
在制造,存储,运输和应用过程中,高能材料(EMs)通常被包围在大气中。因此,深入了解大气对EM衰减的影响就变得很重要。在目前的工作中,分别在真空和包括CO 2,CO,H 2 O在内的九种气氛中,在10个1,3,5-三硝基-1,3,5-三嗪烷(RDX)纳米粒子的系统上进行分子动力学模拟,H 2,N 2,NH 3,O 2,NO和NO 2找出影响。在室温下,RDX与任何气氛之间均未发现明显反应,表明这种影响可忽略不计。然而,在高温下,这九种气氛的影响是可变的,可以分为三种情况。NH 3,CO,NO和NO 2,特别是NH 3,通过消耗RDX及其所产生的中间体来促进RDX的衰变。因为用作加速RDX衰变的催化剂的NO 2被O 2大量消耗,所以该衰变被O 2明显禁止。此外,CO 2,H 2 O,H 2和N的一些禁止作用由于它们的稀释作用,证实了对2的衰变,很少或什至没有涉及它们的反应。此外,NO 2分区主导了RDX衰变的初始步骤,随后发生环断裂。另外,发现通过将RDX中的一个O原子隔开而成为中间产物的ONDNTA的总体指示了RDX在各种气氛中的衰减程度。这项工作提供了对大气对RDX热衰减的影响的全面了解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号