Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The CMG Helicase Bypasses DNA-Protein Cross-Links to Facilitate Their Repair.
Cell ( IF 45.5 ) Pub Date : 2018-12-27 , DOI: 10.1016/j.cell.2018.10.053
Justin L Sparks 1 , Gheorghe Chistol 1 , Alan O Gao 2 , Markus Räschle 3 , Nicolai B Larsen 4 , Matthias Mann 5 , Julien P Duxin 4 , Johannes C Walter 6
Cell ( IF 45.5 ) Pub Date : 2018-12-27 , DOI: 10.1016/j.cell.2018.10.053
Justin L Sparks 1 , Gheorghe Chistol 1 , Alan O Gao 2 , Markus Räschle 3 , Nicolai B Larsen 4 , Matthias Mann 5 , Julien P Duxin 4 , Johannes C Walter 6
Affiliation
|
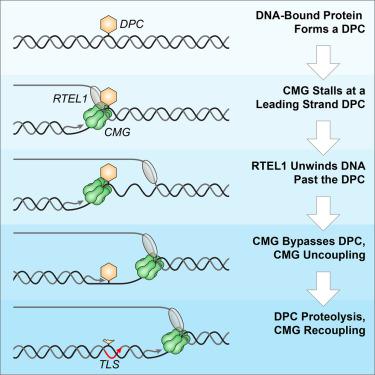
Covalent DNA-protein cross-links (DPCs) impede replication fork progression and threaten genome integrity. Using Xenopus egg extracts, we previously showed that replication fork collision with DPCs causes their proteolysis, followed by translesion DNA synthesis. We show here that when DPC proteolysis is blocked, the replicative DNA helicase CMG (CDC45, MCM2-7, GINS), which travels on the leading strand template, bypasses an intact leading strand DPC. Single-molecule imaging reveals that GINS does not dissociate from CMG during bypass and that CMG slows dramatically after bypass, likely due to uncoupling from the stalled leading strand. The DNA helicase RTEL1 facilitates bypass, apparently by generating single-stranded DNA beyond the DPC. The absence of RTEL1 impairs DPC proteolysis, suggesting that CMG must bypass the DPC to enable proteolysis. Our results suggest a mechanism that prevents inadvertent CMG destruction by DPC proteases, and they reveal CMG's remarkable capacity to overcome obstacles on its translocation strand.
中文翻译:

CMG解旋酶绕过DNA蛋白质交联以促进其修复。
共价DNA-蛋白质交联(DPC)阻碍了复制叉的发展并威胁基因组的完整性。使用非洲爪蟾卵提取物,我们先前表明与DPC的复制叉碰撞会导致其蛋白水解,然后进行病灶DNA合成。我们在此处显示,当DPC蛋白水解受阻时,复制的DNA解旋酶CMG(CDC45,MCM2-7,GINS)在前导链模板上移动,绕过完整的前导链DPC。单分子成像显示,在旁路过程中GINS不会从CMG上解离,并且在旁路后CMG会显着减慢,这可能是由于与停滞的前导链解偶联所致。DNA解旋酶RTEL1显然通过在DPC之外生成单链DNA来促进旁路。RTEL1的缺失会损害DPC的蛋白水解,这表明CMG必须绕过DPC才能进行蛋白水解。
更新日期:2018-12-28
中文翻译:

CMG解旋酶绕过DNA蛋白质交联以促进其修复。
共价DNA-蛋白质交联(DPC)阻碍了复制叉的发展并威胁基因组的完整性。使用非洲爪蟾卵提取物,我们先前表明与DPC的复制叉碰撞会导致其蛋白水解,然后进行病灶DNA合成。我们在此处显示,当DPC蛋白水解受阻时,复制的DNA解旋酶CMG(CDC45,MCM2-7,GINS)在前导链模板上移动,绕过完整的前导链DPC。单分子成像显示,在旁路过程中GINS不会从CMG上解离,并且在旁路后CMG会显着减慢,这可能是由于与停滞的前导链解偶联所致。DNA解旋酶RTEL1显然通过在DPC之外生成单链DNA来促进旁路。RTEL1的缺失会损害DPC的蛋白水解,这表明CMG必须绕过DPC才能进行蛋白水解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号