当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,3-Chlorine Shift to a Vinyl Cation: A Combined Experimental and Theoretical Investigation of the E-Selective Gold(I)-Catalyzed Dimerization of Chloroacetylenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-12-27 , DOI: 10.1021/jacs.8b11501
Mathis Kreuzahler 1 , Alyssa Daniels 1 , Christoph Wölper 1 , Gebhard Haberhauer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-12-27 , DOI: 10.1021/jacs.8b11501
Mathis Kreuzahler 1 , Alyssa Daniels 1 , Christoph Wölper 1 , Gebhard Haberhauer 1
Affiliation
|
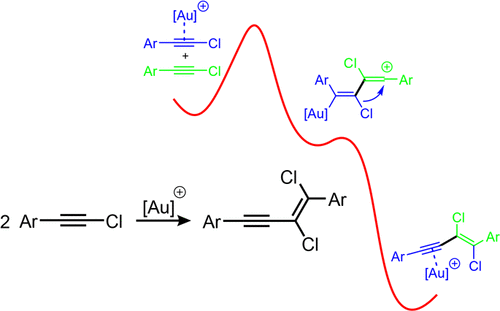
Metal-catalyzed dimerization reactions of terminal acetylenes are well known in the literature. However, only a few examples of the dimerization of halogen-substituted acetylenes are described. The products of the latter metal-catalyzed dimerization are the branched head-to-tail enynes. The formation of the corresponding linear head-to-head enynes has not been reported yet. Herein, we demonstrate by means of quantum chemical methods and experiments that the head-to-head dimerization of chloroarylacetylenes can be achieved via mono gold catalysis. Under the optimized conditions, a clean and complete conversion of the starting materials is observed and the dimeric products are obtained up to 75% NMR yield. A mechanistic investigation of the dimerization reaction reveals that the branched head-to-tail vinyl cation is energetically more stable than the corresponding linear head-to-head cation. However, the latter can rearrange by an unusual 1,3-chlorine shift, resulting in the highly stereoselective formation of the trans product, which corresponds to the gold complex of the head-to-head E-enyne. The activation barrier for this rearrangement is extremely low (ca. 2 kcal/mol). As the mono gold-catalyzed dimerization can be conducted in a preparative scale, this simple synthesis of trans-1,2-dichloroenynes makes the gold(I)-catalyzed head-to-head dimerization of chloroarylacetylenes an attractive method en route to more complex conjugated enyne systems and their congeners.
中文翻译:

1,3-氯转移到乙烯基阳离子:E-选择性金(I)催化氯乙炔二聚的实验和理论研究
末端乙炔的金属催化二聚反应在文献中是众所周知的。然而,仅描述了卤素取代的乙炔二聚的几个例子。后一种金属催化二聚的产物是支链的头尾烯炔。尚未报道相应的线性头对头烯炔的形成。在此,我们通过量子化学方法和实验证明,氯芳基乙炔的头对头二聚反应可以通过单金催化实现。在优化的条件下,观察到起始材料的清洁和完全转化,并获得了高达 75% NMR 产率的二聚产物。二聚反应的机理研究表明,支化的头对尾乙烯基阳离子比相应的线性头对头阳离子在能量上更稳定。然而,后者可以通过不寻常的 1,3-氯位移重新排列,导致反式产物的高度立体选择性形成,这对应于头对头 E-烯炔的金配合物。这种重排的激活势垒极低(约 2 kcal/mol)。由于单金催化的二聚反应可以在制备规模上进行,这种反式-1,2-二氯烯炔的简单合成使金(I)催化的氯芳基乙炔的头对头二聚反应成为一种有吸引力的方法,用于更复杂的共轭烯炔系统及其同系物。后者可以通过不寻常的 1,3-氯位移重排,导致反式产物的高度立体选择性形成,这对应于头对头 E-烯炔的金配合物。这种重排的激活势垒极低(约 2 kcal/mol)。由于单金催化的二聚反应可以在制备规模上进行,这种反式-1,2-二氯烯炔的简单合成使得金(I)催化的氯芳基乙炔头对头二聚反应成为一种有吸引力的方法,用于更复杂的共轭烯炔系统及其同系物。后者可以通过不寻常的 1,3-氯位移重排,导致反式产物的高度立体选择性形成,这对应于头对头 E-烯炔的金配合物。这种重排的激活势垒极低(约 2 kcal/mol)。由于单金催化的二聚反应可以在制备规模上进行,这种反式-1,2-二氯烯炔的简单合成使金(I)催化的氯芳基乙炔的头对头二聚反应成为一种有吸引力的方法,用于更复杂的共轭烯炔系统及其同系物。
更新日期:2018-12-27
中文翻译:

1,3-氯转移到乙烯基阳离子:E-选择性金(I)催化氯乙炔二聚的实验和理论研究
末端乙炔的金属催化二聚反应在文献中是众所周知的。然而,仅描述了卤素取代的乙炔二聚的几个例子。后一种金属催化二聚的产物是支链的头尾烯炔。尚未报道相应的线性头对头烯炔的形成。在此,我们通过量子化学方法和实验证明,氯芳基乙炔的头对头二聚反应可以通过单金催化实现。在优化的条件下,观察到起始材料的清洁和完全转化,并获得了高达 75% NMR 产率的二聚产物。二聚反应的机理研究表明,支化的头对尾乙烯基阳离子比相应的线性头对头阳离子在能量上更稳定。然而,后者可以通过不寻常的 1,3-氯位移重新排列,导致反式产物的高度立体选择性形成,这对应于头对头 E-烯炔的金配合物。这种重排的激活势垒极低(约 2 kcal/mol)。由于单金催化的二聚反应可以在制备规模上进行,这种反式-1,2-二氯烯炔的简单合成使金(I)催化的氯芳基乙炔的头对头二聚反应成为一种有吸引力的方法,用于更复杂的共轭烯炔系统及其同系物。后者可以通过不寻常的 1,3-氯位移重排,导致反式产物的高度立体选择性形成,这对应于头对头 E-烯炔的金配合物。这种重排的激活势垒极低(约 2 kcal/mol)。由于单金催化的二聚反应可以在制备规模上进行,这种反式-1,2-二氯烯炔的简单合成使得金(I)催化的氯芳基乙炔头对头二聚反应成为一种有吸引力的方法,用于更复杂的共轭烯炔系统及其同系物。后者可以通过不寻常的 1,3-氯位移重排,导致反式产物的高度立体选择性形成,这对应于头对头 E-烯炔的金配合物。这种重排的激活势垒极低(约 2 kcal/mol)。由于单金催化的二聚反应可以在制备规模上进行,这种反式-1,2-二氯烯炔的简单合成使金(I)催化的氯芳基乙炔的头对头二聚反应成为一种有吸引力的方法,用于更复杂的共轭烯炔系统及其同系物。































 京公网安备 11010802027423号
京公网安备 11010802027423号