当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and stereochemistry of new naphth[1,3]oxazino[3,2-a]benzazepine and naphth[1,3]oxazino[3,2-e]thienopyridine derivatives
Tetrahedron ( IF 2.1 ) Pub Date : 2016-03-18 06:45:04 Petra Barta, István Szatmári, Ferenc Fülöp, Matthias Heydenreich, Andreas Koch, Erich Kleinpeter
Tetrahedron ( IF 2.1 ) Pub Date : 2016-03-18 06:45:04 Petra Barta, István Szatmári, Ferenc Fülöp, Matthias Heydenreich, Andreas Koch, Erich Kleinpeter
|
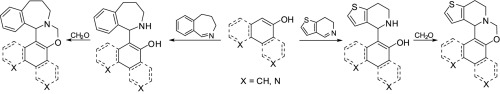
Through the reactions of 1- or 2-naphthol and 4,5-dihydro-3H-benz[c]azepine or 6,7-dihydrothieno[3,2-c]pyridine, new aminonaphthol derivatives were prepared. The syntheses were extended by using N-containing naphthol analogues such as 5-hydroxyisoquinoline and 6-hydroxyquinoline. The ring closures of the novel bifunctional compounds were also achieved, resulting in new naphth[2,1-e][1,3]oxazines, naphth[1,2-e][1,3]oxazines, isoquinolino[5,6-e][1,3]oxazines and quinolino[5,6-e][1,3]oxazines. 1H NMR spectra of of the target heterocycles 16, 20 and 21 were sufficiently resolved to indentify the present stereochemistry; therefore, beside computed structures, spatial experimental (dipolar coupling − NOE) and computed (ring current effect of the naphthyl moiety − TSNMRS) NMR studies were employed. The studied heterocycles exist exclusively as S(14b),R(N), R(14b),S(N), and S(16b)S(N) isomers, respectively. The flexible moieties of the studied compounds prefer.
中文翻译:

新型萘[1,3]恶嗪[3,2-a]苯并enza庚因和萘[1,3]恶嗪[3,2-e]噻吩并吡啶衍生物的合成和立体化学
通过1-或2-萘酚与4,5-二氢-3H-苯并[c] a庚因或6,7-二氢噻吩并[3,2-c]吡啶的反应,制备了新的氨基萘酚衍生物。通过使用含氮的萘酚类似物(例如5-羟基异喹啉和6-羟基喹啉)扩展合成。还实现了新型双功能化合物的闭环,产生了新的萘并[2,1-e] [1,3]恶嗪,萘并[1,2-e] [1,3]恶嗪,异喹啉[5,6]。 -e] [1,3]恶嗪和喹啉基[5,6-e] [1,3]恶嗪。1个目标杂环16、20和21的1 H NMR光谱得到了足够的分辨,以鉴定本发明的立体化学。因此,除了计算结构,还进行了空间实验(偶极耦合-NOE)和计算(萘基部分的环电流效应-TSNMRS)NMR研究。所研究的杂环分别以S(14b),R(N),R(14b),S(N)和S(16b)S(N)异构体形式存在。所研究化合物的柔性部分是优选的。
更新日期:2016-03-18
中文翻译:

新型萘[1,3]恶嗪[3,2-a]苯并enza庚因和萘[1,3]恶嗪[3,2-e]噻吩并吡啶衍生物的合成和立体化学
通过1-或2-萘酚与4,5-二氢-3H-苯并[c] a庚因或6,7-二氢噻吩并[3,2-c]吡啶的反应,制备了新的氨基萘酚衍生物。通过使用含氮的萘酚类似物(例如5-羟基异喹啉和6-羟基喹啉)扩展合成。还实现了新型双功能化合物的闭环,产生了新的萘并[2,1-e] [1,3]恶嗪,萘并[1,2-e] [1,3]恶嗪,异喹啉[5,6]。 -e] [1,3]恶嗪和喹啉基[5,6-e] [1,3]恶嗪。1个目标杂环16、20和21的1 H NMR光谱得到了足够的分辨,以鉴定本发明的立体化学。因此,除了计算结构,还进行了空间实验(偶极耦合-NOE)和计算(萘基部分的环电流效应-TSNMRS)NMR研究。所研究的杂环分别以S(14b),R(N),R(14b),S(N)和S(16b)S(N)异构体形式存在。所研究化合物的柔性部分是优选的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号