当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Catalysis in Carboxylic Acid Esterification: Modeling of Single- and Multi-Acid Esterification
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-01-10 , DOI: 10.1021/acs.iecr.8b04666 Daniela Painer 1 , Susanne Lux 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-01-10 , DOI: 10.1021/acs.iecr.8b04666 Daniela Painer 1 , Susanne Lux 1
Affiliation
|
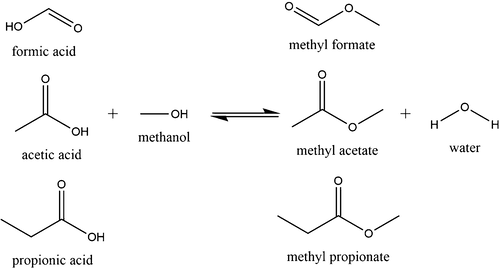
Downstream processing in the biorefinery is often faced with multicomponent process streams containing carboxylic acids and alcohols. Isolation of carboxylic acids may be simplified by direct esterification with the alcohol present in the process stream. However, uncatalyzed esterification is generally slow at moderate temperature. In multicomponent mixtures, though, stronger carboxylic acids act as catalysts for the esterification of weaker carboxylic acids. Single-acid esterification of formic acid, acetic acid, and propionic acid with methanol were investigated, and a kinetic model was developed considering the intramolecular catalytic effect through acid dissociation. With the kinetic constants derived from single-acid esterification, ester formation in multi-acid esterification was predicted and verified experimentally with a maximum sum of mean square error of 8.70 × 10–4. In multi-acid esterification, the catalytic influence of formic acid on acetic acid and propionic acid esterification was shown. Due to higher equilibrium constants transesterification of methyl formate to methyl acetate and methyl propionate, respectively, is observed and predicted by the model.
中文翻译:

羧酸酯化中的分子内催化:单酸和多酸酯化的建模
生物精炼厂的下游处理通常面临着包含羧酸和醇的多组分处理流。通过与工艺物流中存在的醇直接酯化可以简化羧酸的分离。然而,未催化的酯化通常在中等温度下缓慢。但是,在多组分混合物中,较强的羧酸充当弱羧酸酯化的催化剂。研究了甲酸,乙酸和丙酸与甲醇的单酸酯化反应,并考虑了通过酸解离的分子内催化作用,建立了动力学模型。利用单酸酯化反应得到的动力学常数,–4。在多酸酯化中,显示了甲酸对乙酸和丙酸酯化的催化作用。由于较高的平衡常数,该模型观察到并预测了甲酸甲酯分别酯交换为乙酸甲酯和丙酸甲酯。
更新日期:2019-01-11
中文翻译:

羧酸酯化中的分子内催化:单酸和多酸酯化的建模
生物精炼厂的下游处理通常面临着包含羧酸和醇的多组分处理流。通过与工艺物流中存在的醇直接酯化可以简化羧酸的分离。然而,未催化的酯化通常在中等温度下缓慢。但是,在多组分混合物中,较强的羧酸充当弱羧酸酯化的催化剂。研究了甲酸,乙酸和丙酸与甲醇的单酸酯化反应,并考虑了通过酸解离的分子内催化作用,建立了动力学模型。利用单酸酯化反应得到的动力学常数,–4。在多酸酯化中,显示了甲酸对乙酸和丙酸酯化的催化作用。由于较高的平衡常数,该模型观察到并预测了甲酸甲酯分别酯交换为乙酸甲酯和丙酸甲酯。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号