当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-((Hetera)cyclobutyl)azetidines, "Stretched" Analogues of Piperidine, Piperazine, and Morpholine: Advanced Building Blocks for Drug Discovery.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-12-24 00:00:00 , DOI: 10.1021/acs.joc.8b02822 Illia O Feskov 1, 2 , Anton V Chernykh 1 , Yuliya O Kuchkovska 1, 3 , Constantin G Daniliuc 4 , Ivan S Kondratov 1, 2 , Oleksandr O Grygorenko 1, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-12-24 00:00:00 , DOI: 10.1021/acs.joc.8b02822 Illia O Feskov 1, 2 , Anton V Chernykh 1 , Yuliya O Kuchkovska 1, 3 , Constantin G Daniliuc 4 , Ivan S Kondratov 1, 2 , Oleksandr O Grygorenko 1, 3
Affiliation
|
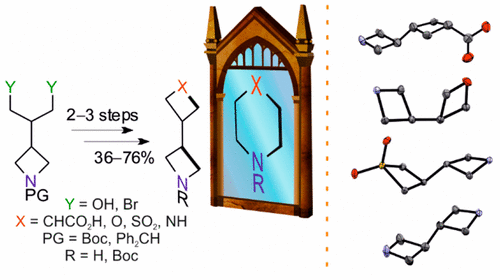
Four 3-((hetera)cyclobutyl)azetidine-based isosteres of piperidine, piperazine, and morpholine were designed and synthesized on up to gram scale. The key step of the synthetic sequence included cyclization of N-protected 2-(azetidin-3-yl)propane-1,3-diol or the corresponding 1,3-dibromide. X-ray diffraction studies of the products obtained, followed by exit vector plot analysis of their molecular geometry, demonstrated their larger size and increased conformational flexibility as compared to the parent heterocycles and confirmed their potential utility as building blocks for lead optimization programs.
中文翻译:

3-((杂)环丁基)氮杂环丁烷,哌啶,哌嗪和吗啉的“拉伸”类似物:药物发现的高级构件。
设计并合成了4个基于3-((杂)环丁基)氮杂环丁烷的哌啶,哌嗪和吗啉等位异构体,合成量达到了克级。合成序列的关键步骤包括将N保护的2-(氮杂环丁烷-3-基)丙烷-1,3-二醇或相应的1,3-二溴化物环化。对所得产物进行X射线衍射研究,然后对其分子几何结构进行出口向量图分析,证明了与母体杂环相比,它们的尺寸更大,构象柔韧性更高,并证实了其潜在的实用性,可作为潜在客户优化程序的基础。
更新日期:2018-12-24
中文翻译:

3-((杂)环丁基)氮杂环丁烷,哌啶,哌嗪和吗啉的“拉伸”类似物:药物发现的高级构件。
设计并合成了4个基于3-((杂)环丁基)氮杂环丁烷的哌啶,哌嗪和吗啉等位异构体,合成量达到了克级。合成序列的关键步骤包括将N保护的2-(氮杂环丁烷-3-基)丙烷-1,3-二醇或相应的1,3-二溴化物环化。对所得产物进行X射线衍射研究,然后对其分子几何结构进行出口向量图分析,证明了与母体杂环相比,它们的尺寸更大,构象柔韧性更高,并证实了其潜在的实用性,可作为潜在客户优化程序的基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号