当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Faradaic Efficiency of Oxygen Evolution on Reduced HxWO3 Photoelectrodes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-01-08 , DOI: 10.1021/acs.jpcc.8b11777
Andrew G. Breuhaus-Alvarez 1 , John L. DiMeglio 1 , Joshua J. Cooper 1 , Charles R. Lhermitte 1 , Bart M. Bartlett 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-01-08 , DOI: 10.1021/acs.jpcc.8b11777
Andrew G. Breuhaus-Alvarez 1 , John L. DiMeglio 1 , Joshua J. Cooper 1 , Charles R. Lhermitte 1 , Bart M. Bartlett 1
Affiliation
|
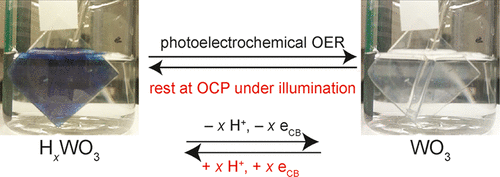
Tungsten oxide (WO3) electrodes were synthesized by spin-coating an ammonium metatungstate sol. Instability in photocurrent during water oxidation applications has previously been attributed to formation of destructive peroxide intermediates. Under constant illumination, repeated cycles of poising WO3 electrodes at 0.98 V vs Ag/AgCl in pH 1 sulfate solution followed by measuring the open-circuit potential for several hours show reversibility in the photocurrent decay. This behavior is attributed to photochromic HxWO3 generated at low concentration within the electrode, which serves to increase the donor density. The Mott–Schottky analysis of electrochemical impedance spectroscopy measurements on WO3 electrodes before and after performing the oxygen-evolution reaction (OER) exhibits a decrease in donor density from 2.8 × 1022 to 6.0 × 1021 cm–3 with a corresponding 110 mV positive shift in the flat-band potential, indicative of tungsten oxidation during the OER. Tungsten oxidation is corroborated by a decrease in W5+ signal in the X-ray photoelectron spectroscopy data. Measuring the OER rate by gas chromatography during water oxidation shows concurrent recovery of catalytic activity after resting at open circuit under illumination, illustrating the key role of HxWO3 during photoelectrocatalysis.
中文翻译:

还原的H x WO 3光电极上析氧的动力学和法拉第效率
通过旋涂偏钨酸铵溶胶合成了氧化钨(WO 3)电极。在水氧化应用期间光电流的不稳定性先前已归因于破坏性的过氧化物中间体的形成。在恒定的照明下,将WO 3电极在pH 1的硫酸盐溶液中相对于Ag / AgCl在0.98 V下放置的重复循环,然后测量开路电势数小时,显示出光电流衰减的可逆性。该行为归因于在电极内以低浓度产生的光致变色H x WO 3,其用于增加施主密度。WO 3上电化学阻抗谱测量的Mott-Schottky分析进行放氧反应(OER)之前和之后的电极显示出施主密度从2.8×10 22降低至6.0×10 21 cm –3,且相应的平带电势为110 mV正移,表明钨被氧化在OER期间。X射线光电子能谱数据中W 5+信号的减少证实了钨的氧化。在水氧化过程中通过气相色谱法测量的OER速率表明,在光照下处于开路状态后,催化活性会同时恢复,这说明了H x WO 3在光电催化过程中的关键作用。
更新日期:2019-01-09
中文翻译:

还原的H x WO 3光电极上析氧的动力学和法拉第效率
通过旋涂偏钨酸铵溶胶合成了氧化钨(WO 3)电极。在水氧化应用期间光电流的不稳定性先前已归因于破坏性的过氧化物中间体的形成。在恒定的照明下,将WO 3电极在pH 1的硫酸盐溶液中相对于Ag / AgCl在0.98 V下放置的重复循环,然后测量开路电势数小时,显示出光电流衰减的可逆性。该行为归因于在电极内以低浓度产生的光致变色H x WO 3,其用于增加施主密度。WO 3上电化学阻抗谱测量的Mott-Schottky分析进行放氧反应(OER)之前和之后的电极显示出施主密度从2.8×10 22降低至6.0×10 21 cm –3,且相应的平带电势为110 mV正移,表明钨被氧化在OER期间。X射线光电子能谱数据中W 5+信号的减少证实了钨的氧化。在水氧化过程中通过气相色谱法测量的OER速率表明,在光照下处于开路状态后,催化活性会同时恢复,这说明了H x WO 3在光电催化过程中的关键作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号