当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Iron Porphyrins for Probing the Differences in the Electronic Structures of Heme a3, Heme d, and Heme d1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-12-21 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02063 Sk Amanullah 1 , Paramita Saha 1 , Rajat Saha 1 , Abhishek Dey 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-12-21 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02063 Sk Amanullah 1 , Paramita Saha 1 , Rajat Saha 1 , Abhishek Dey 1
Affiliation
|
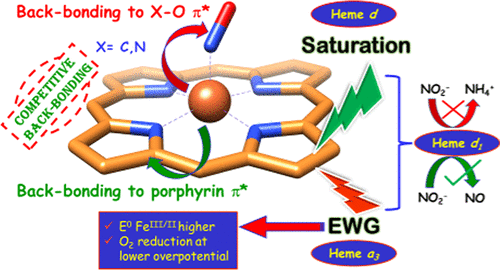
A variety of heme derivatives are pervasive in nature, having different architectures that are complementary to their function. Herein, we report the synthesis of a series of iron porphyrinoids, which bear electron-withdrawing groups and/or are saturated at the β-pyrrolic position, mimicking the structural variation of naturally occurring hemes. The effects of the aforementioned factors were systematically studied using a combination of electrochemistry, spectroscopy, and theoretical calculations with the carbon monoxide (CO) and nitric oxide (NO) adducts of these iron porphyinoids. The reduction potentials of iron porphyrinoids vary over several hundreds of millivolts, and the X–O (X = C, N) vibrations of the adducts vary over 10–15 cm–1. Density functional theory calculations indicate that the presence of electron-withdrawing groups and saturation of the pyrrole ring lowers the π*-acceptor orbital energies of the macrocycle, which, in turn, attenuates the bonding of iron to CO and NO. A hypothesis has been presented as to why cytochrome c containing nitrite reductases and cytochrome cd1 containing nitrite reductases follow different mechanistic pathways of nitrite reduction. This study also helps to rationalize the choice of heme a3 and not the most abundant heme b cofactor in cytochrome c oxidase.
中文翻译:

合成铁卟啉用于探测血红素a 3,血红素d和血红素d 1的电子结构的差异
各种血红素衍生物本质上是普遍存在的,具有与其功能互补的不同结构。在本文中,我们报道了一系列铁卟啉类化合物的合成,这些类卟啉类化合物具有吸电子基团和/或在β-吡咯位置上饱和,模仿了天然血红素的结构变异。利用电化学,光谱学和理论计算结合这些铁卟啉类化合物的一氧化碳(CO)和一氧化氮(NO)加合物,系统地研究了上述因素的影响。铁卟啉类化合物的还原电位在几百毫伏内变化,加合物的X–O(X = C,N)振动在10–15 cm –1之间变化。密度泛函理论计算表明,吸电子基团的存在和吡咯环的饱和降低了大环的π*受体轨道能,从而削弱了铁与CO和NO的键合。已经提出了关于为什么含有亚硝酸还原酶的细胞色素c和含有亚硝酸还原酶的细胞色素cd 1遵循不同的亚硝酸还原机理的假设。这项研究还有助于合理化选择血红素a 3而不是细胞色素c氧化酶中最丰富的血红素b辅因子。
更新日期:2018-12-21
中文翻译:

合成铁卟啉用于探测血红素a 3,血红素d和血红素d 1的电子结构的差异
各种血红素衍生物本质上是普遍存在的,具有与其功能互补的不同结构。在本文中,我们报道了一系列铁卟啉类化合物的合成,这些类卟啉类化合物具有吸电子基团和/或在β-吡咯位置上饱和,模仿了天然血红素的结构变异。利用电化学,光谱学和理论计算结合这些铁卟啉类化合物的一氧化碳(CO)和一氧化氮(NO)加合物,系统地研究了上述因素的影响。铁卟啉类化合物的还原电位在几百毫伏内变化,加合物的X–O(X = C,N)振动在10–15 cm –1之间变化。密度泛函理论计算表明,吸电子基团的存在和吡咯环的饱和降低了大环的π*受体轨道能,从而削弱了铁与CO和NO的键合。已经提出了关于为什么含有亚硝酸还原酶的细胞色素c和含有亚硝酸还原酶的细胞色素cd 1遵循不同的亚硝酸还原机理的假设。这项研究还有助于合理化选择血红素a 3而不是细胞色素c氧化酶中最丰富的血红素b辅因子。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号