当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Topologically Distinct Modified Peptide with Multiple Bicyclic Core Motifs Expands the Diversity of Microviridin-Like Peptides.
ChemBioChem ( IF 2.6 ) Pub Date : 2019-03-04 , DOI: 10.1002/cbic.201800678 Heejin Roh 1 , Yeji Han 1 , Hyunbin Lee 1 , Seokhee Kim 1
ChemBioChem ( IF 2.6 ) Pub Date : 2019-03-04 , DOI: 10.1002/cbic.201800678 Heejin Roh 1 , Yeji Han 1 , Hyunbin Lee 1 , Seokhee Kim 1
Affiliation
|
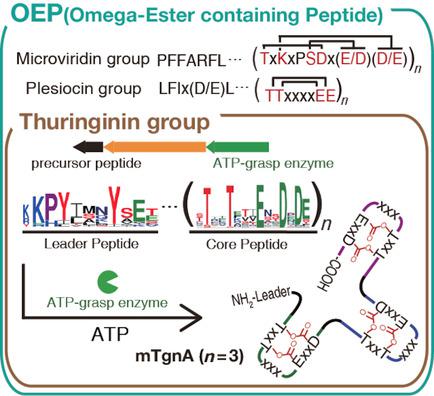
Microviridins are ribosomally synthesized and post-translationally modified peptides (RiPPs) that contain multiple intramolecular ω-ester or ω-amide crosslinks between two side chains in peptides. This type of the side-to-side macrocyclization may generate diverse structures with distinct topology and ring sizes, but the majority of the microviridin-like RiPPs present only a single consensus sequence with a tricyclic architecture. Here, we expanded the natural diversity of the microviridin-like modified peptides by determining the crosslinking connectivity of a new modified peptide, mTgnA and its homologous RiPPs, which we named the thuringinin group. Members of the thuringinin group have core motifs with a distinct consensus sequence, which is transformed to a novel hairpin-like bicyclic structure by the cognate ATP-grasp enzyme. We suggest that the microviridin-like RiPPs naturally have novel sequences and architectures beyond those found in microviridins and comprise a larger RiPP family, termed omega-ester containing peptides (OEPs).
中文翻译:

具有多个双环核心基序的拓扑不同的修饰肽扩大了微病毒素样肽的多样性。
微病毒素是核糖体合成的和翻译后修饰的肽(RiPP),在肽的两个侧链之间包含多个分子内ω-酯或ω-酰胺交联键。这种类型的侧面到侧面大环化可能会生成具有不同拓扑和环大小的各种结构,但是大多数微病毒素样RiPPs仅呈现具有三环结构的单个共有序列。在这里,我们通过确定新的修饰肽mTgnA及其同源RiPPs的交联连接性(我们将其命名为图林汀组),扩大了微病毒素样修饰肽的自然多样性。苏林素族的成员具有核心基序,具有不同的共有序列,该序列通过关联的ATP抓握酶转化为新的发夹状双环结构。
更新日期:2019-03-04
中文翻译:

具有多个双环核心基序的拓扑不同的修饰肽扩大了微病毒素样肽的多样性。
微病毒素是核糖体合成的和翻译后修饰的肽(RiPP),在肽的两个侧链之间包含多个分子内ω-酯或ω-酰胺交联键。这种类型的侧面到侧面大环化可能会生成具有不同拓扑和环大小的各种结构,但是大多数微病毒素样RiPPs仅呈现具有三环结构的单个共有序列。在这里,我们通过确定新的修饰肽mTgnA及其同源RiPPs的交联连接性(我们将其命名为图林汀组),扩大了微病毒素样修饰肽的自然多样性。苏林素族的成员具有核心基序,具有不同的共有序列,该序列通过关联的ATP抓握酶转化为新的发夹状双环结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号