当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Elucidation of Gold(I)-Catalyzed Oxidation of a Propargylic Alcohol by a N-Oxide in the Presence of an Imine Using DFT Calculations
Organometallics ( IF 2.5 ) Pub Date : 2018-12-19 , DOI: 10.1021/acs.organomet.8b00808
Fatemeh Zarkoob 1 , Alireza Ariafard 1, 2
Organometallics ( IF 2.5 ) Pub Date : 2018-12-19 , DOI: 10.1021/acs.organomet.8b00808
Fatemeh Zarkoob 1 , Alireza Ariafard 1, 2
Affiliation
|
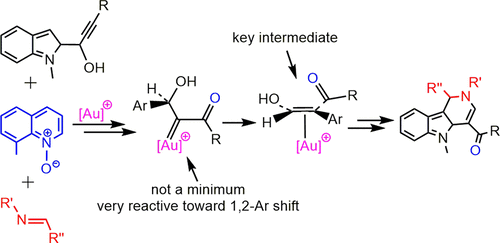
Density functional theory (DFT) calculations were utilized to investigate the mechanism of the oxidation of an indolyl propargylic alcohol by a N-oxide in the presence of an imine and a gold(I) catalyst. The catalytic reaction is proposed to start from regioselective oxidation of the gold(I)-activated alkyne dictated by a hydrogen bond interaction between the OH group of the propargylic alcohol and the N-oxide. This oxidation was expected to give an α-carbonyl gold carbene complex. In contrast to this expectation, our calculations showed that the corresponding carbene is not a local minimum and the complex undergoes a very fast 1,2 aryl shift to form an alkene complex. Subsequently, an imine is added to the ensuing alkene complex to give an iminium cation from which a cycloaddition process occurs and an indolium is formed. Finally, an N-oxide deprotonates the indolium complex and affords an intermediate which is significantly reactive toward water elimination. Our calculations indicate that the 1,2-aryl shift in α-carbonyl gold carbene complexes is decelerated if the aryl is substituted by an electron-withdrawing group. At the end, we investigated the stability of different gold carbene complexes and found that the identity of the carbene is the determinant of how these carbenes are trapped.
中文翻译:

使用DFT计算方法在亚胺存在下用N-氧化物催化金(I)催化丙醇的氧化机理
密度泛函理论(DFT)计算用于研究亚胺和金(I)催化剂存在下N-氧化物氧化吲哚基炔丙醇的机理。提出催化反应从金(I)活化的炔的区域选择性氧化开始,该炔由炔丙基醇的OH基团和N-氧化物之间的氢键相互作用决定。预期该氧化将产生α-羰基金卡宾络合物。与此预期相反,我们的计算表明相应的卡宾不是局部最小值,并且该配合物经历非常快的1,2芳基移位,形成烯烃配合物。随后,将亚胺添加到随后的烯烃络合物中,以产生亚胺阳离子,从该亚胺阳离子发生环加成过程并形成吲哚。最后,N-氧化物使吲哚络合物去质子化,并提供对除水有显着反应的中间体。我们的计算表明,如果芳基被吸电子基团取代,则α-羰基金卡宾络合物中的1,2-芳基转移将被减速。最后,我们研究了不同的金卡宾络合物的稳定性,并发现卡宾的身份是如何捕获这些卡宾的决定因素。
更新日期:2018-12-19
中文翻译:

使用DFT计算方法在亚胺存在下用N-氧化物催化金(I)催化丙醇的氧化机理
密度泛函理论(DFT)计算用于研究亚胺和金(I)催化剂存在下N-氧化物氧化吲哚基炔丙醇的机理。提出催化反应从金(I)活化的炔的区域选择性氧化开始,该炔由炔丙基醇的OH基团和N-氧化物之间的氢键相互作用决定。预期该氧化将产生α-羰基金卡宾络合物。与此预期相反,我们的计算表明相应的卡宾不是局部最小值,并且该配合物经历非常快的1,2芳基移位,形成烯烃配合物。随后,将亚胺添加到随后的烯烃络合物中,以产生亚胺阳离子,从该亚胺阳离子发生环加成过程并形成吲哚。最后,N-氧化物使吲哚络合物去质子化,并提供对除水有显着反应的中间体。我们的计算表明,如果芳基被吸电子基团取代,则α-羰基金卡宾络合物中的1,2-芳基转移将被减速。最后,我们研究了不同的金卡宾络合物的稳定性,并发现卡宾的身份是如何捕获这些卡宾的决定因素。

































 京公网安备 11010802027423号
京公网安备 11010802027423号