当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tailoring Photophysical Processes of Perylene-Based Light Harvesting Antenna Systems with Molecular Structure and Solvent Polarity
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-12-31 , DOI: 10.1021/acs.jpcc.8b08503 Damla Inan , Rajeev K. Dubey , Wolter F. Jager , Ferdinand C. Grozema
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-12-31 , DOI: 10.1021/acs.jpcc.8b08503 Damla Inan , Rajeev K. Dubey , Wolter F. Jager , Ferdinand C. Grozema
|
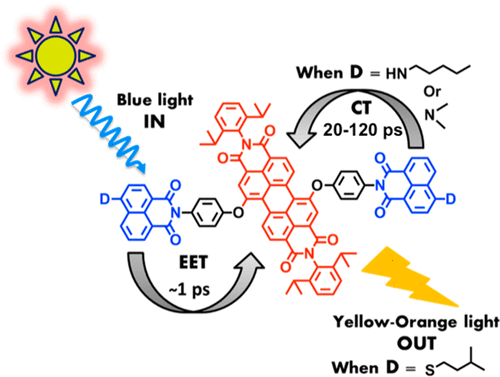
The excited-state dynamics of perylene-based bichromophoric light harvesting antenna systems has been tailored by systematic modification of the molecular structure and by using solvents of increasing polarity in the series toluene, chloroform, and benzonitrile. The antenna systems consist of blue light absorbing naphthalene monoimide (NMI) energy donors (D1, D2, and D3) and the perylene derived green light absorbing energy acceptor moieties, 1,7-perylene-3,4,9,10-tetracarboxylic tetrabutylester (A1), 1,7-perylene-3,4,9,10-tetracarboxylic monoimide dibutylester (A2), and 1,7-perylene-3,4,9,10-tetracarboxylic bisimide (A3). The design of these antenna systems is such that all exhibit ultrafast excitation energy transfer (EET) from the excited donor to the acceptor, due to the effective matching of optical properties of the constituent chromophores. At the same time, electron transfer from the donor to the excited acceptor unit has been limited by the use of a rigid and nonconjugated phenoxy bridge to link the donor and acceptor components. The antenna molecules D1A1, D1A2, and D1A3, which bear the least electron-rich energy donor, isopentylthio-substituted NMI D1, exhibited ultrafast EET (τEET ∼ 1 ps) but no charge transfer and, resultantly, emitted a strong yellow-orange acceptor fluorescence upon excitation of the donor. The other antenna molecules D2A2, D2A3, and D3A3, which bear electron-rich energy donors, the amino-substituted NMIs D2 and D3, exhibited ultrafast energy transfer that was followed by a slower (ca. 20–2000 ps) electron transfer from the donor to the excited acceptor. This charge transfer quenched the acceptor fluorescence to an extent determined by molecular structure and solvent polarity. These antenna systems mimic the primary events occurring in the natural photosynthesis, i.e., energy capture, efficient energy funneling toward the central chromophore, and finally charge separation, and are suitable building blocks for achieving artificial photosynthesis, because of their robustness and favorable and tunable photophysical properties.
中文翻译:

基于分子结构和溶剂极性的Per基光收集天线系统的光物理过程定制
通过分子结构的系统修饰和在甲苯,氯仿和苄腈系列中使用增加极性的溶剂,可以定制per基双发色光收集天线系统的激发态动力学。天线系统由吸收蓝光的萘单酰亚胺(NMI)能量供体(D1,D2和D3)和the衍生的绿光吸收能量受体部分,1,7-per-3,4,9,10-四羧酸四丁酯组成(A1),1,7-per-3,4,9,10-四羧酸单酰亚胺二丁酯(A2)和1,7-per-3,4,9,10-四羧酸双酰亚胺(A3))。这些天线系统的设计使得,由于组成发色团的光学特性的有效匹配,所有天线系统均表现出从受激施主到受主的超快激发能量转移(EET)。同时,通过使用刚性的和非共轭的苯氧基桥来连接供体和受体的成分,限制了从供体到受激受体单元的电子转移。带有最少电子富集能量供体的异戊硫基取代的NMI D1的天线分子D1A1,D1A2和D1A3表现出超快的EET(τEET约1 ps),但没有电荷转移,因此在供体激发时发出了强烈的橙橙色受体荧光。带有富电子供体的其他天线分子D2A2,D2A3和D3A3为氨基取代的NMI D2和D3,表现出超快的能量转移,随后是较慢的(约20–2000 ps)电子从供体转移到受激受体。这种电荷转移将受体荧光猝灭到由分子结构和溶剂极性决定的程度。这些天线系统模仿自然光合作用中发生的主要事件,即能量捕获,向中心发色团的有效能量漏斗以及最终的电荷分离,并且由于其坚固性和有利的且可调节的光物理特性,因此是实现人工光合作用的合适构建基块。特性。
更新日期:2019-01-01
中文翻译:

基于分子结构和溶剂极性的Per基光收集天线系统的光物理过程定制
通过分子结构的系统修饰和在甲苯,氯仿和苄腈系列中使用增加极性的溶剂,可以定制per基双发色光收集天线系统的激发态动力学。天线系统由吸收蓝光的萘单酰亚胺(NMI)能量供体(D1,D2和D3)和the衍生的绿光吸收能量受体部分,1,7-per-3,4,9,10-四羧酸四丁酯组成(A1),1,7-per-3,4,9,10-四羧酸单酰亚胺二丁酯(A2)和1,7-per-3,4,9,10-四羧酸双酰亚胺(A3))。这些天线系统的设计使得,由于组成发色团的光学特性的有效匹配,所有天线系统均表现出从受激施主到受主的超快激发能量转移(EET)。同时,通过使用刚性的和非共轭的苯氧基桥来连接供体和受体的成分,限制了从供体到受激受体单元的电子转移。带有最少电子富集能量供体的异戊硫基取代的NMI D1的天线分子D1A1,D1A2和D1A3表现出超快的EET(τEET约1 ps),但没有电荷转移,因此在供体激发时发出了强烈的橙橙色受体荧光。带有富电子供体的其他天线分子D2A2,D2A3和D3A3为氨基取代的NMI D2和D3,表现出超快的能量转移,随后是较慢的(约20–2000 ps)电子从供体转移到受激受体。这种电荷转移将受体荧光猝灭到由分子结构和溶剂极性决定的程度。这些天线系统模仿自然光合作用中发生的主要事件,即能量捕获,向中心发色团的有效能量漏斗以及最终的电荷分离,并且由于其坚固性和有利的且可调节的光物理特性,因此是实现人工光合作用的合适构建基块。特性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号