当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of the Reverse Water Gas Shift Reaction on Copper Modified β-Mo2C(001) Surfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-12-31 , DOI: 10.1021/acs.jpcc.8b09884 Huijuan Jing 1, 2 , Qiaohong Li 1 , Jian Wang 1 , Diwen Liu 1, 2 , Kechen Wu 1, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-12-31 , DOI: 10.1021/acs.jpcc.8b09884 Huijuan Jing 1, 2 , Qiaohong Li 1 , Jian Wang 1 , Diwen Liu 1, 2 , Kechen Wu 1, 3
Affiliation
|
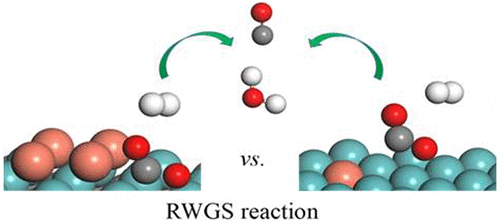
The reverse water gas shift (RWGS) reaction has attracted great attention in recent years. It is well-known that supported catalysts, especially single-atom catalysts (SACs), exhibit good catalytic activity in many reactions. Thus, we designed the single-atom catalyst (SAC) Cu@Mo2C(001) and the smallest copper cluster catalyst Cu4@Mo2C(001) for the RWGS reaction. In this study, density functional theory (DFT) calculations were used to explore the reaction mechanisms of the RWGS reaction on the surfaces of Cu@Mo2C(001) and Cu4@Mo2C(001). The dissociative adsorption of H2 on these two surfaces is barrier-free and highly exothermic, which is beneficial to the RWGS reaction. Importantly, three possible mechanisms—the COOH mechanism, HCOO mechanism, and redox mechanism—have been discussed. The results illustrated that the redox mechanism is the most feasible pathway among the Cu@Mo2C(001) and Cu4@Mo2C(001) surfaces. By comparing the activation barrier of the rate-limiting step of the redox mechanism on the two surfaces, the results showed that the activation barrier of the rate-limiting step on the Cu4@Mo2C(001) surface is smaller, which is more conducive to the progress of the RWGS reaction. Therefore, the tactic of introducing non-noble copper could be a promising way to design highly efficient catalysts.
中文翻译:

修改于铜反向水煤气变换反应的理论研究β-沫2 C(001)面
近年来,逆水煤气变换(RWGS)反应引起了极大的关注。众所周知,载体上的催化剂,特别是单原子催化剂(SAC),在许多反应中都表现出良好的催化活性。因此,我们设计了用于RWGS反应的单原子催化剂(SAC)Cu @ Mo 2 C(001)和最小的铜簇催化剂Cu 4 @Mo 2 C(001)。在这项研究中,使用密度泛函理论(DFT)计算来探索RWGS反应在Cu @ Mo 2 C(001)和Cu 4 @Mo 2 C(001)表面的反应机理。H 2的解离吸附在这两个表面上的碳原子是无障碍的并且高度放热,这对RWGS反应是有利的。重要的是,已经讨论了三种可能的机制-COOH机制,HCOO机制和氧化还原机制。结果表明,氧化还原机制是Cu @ Mo 2 C(001)和Cu 4 @Mo 2 C(001)表面之间最可行的途径。通过比较两个表面上氧化还原机理的限速步骤的激活势垒,结果表明限速步骤对Cu 4 @Mo 2的激活势垒C(001)表面较小,这更有利于RWGS反应的进行。因此,引入非贵金属铜的策略可能是设计高效催化剂的一种有前途的方法。
更新日期:2019-01-01
中文翻译:

修改于铜反向水煤气变换反应的理论研究β-沫2 C(001)面
近年来,逆水煤气变换(RWGS)反应引起了极大的关注。众所周知,载体上的催化剂,特别是单原子催化剂(SAC),在许多反应中都表现出良好的催化活性。因此,我们设计了用于RWGS反应的单原子催化剂(SAC)Cu @ Mo 2 C(001)和最小的铜簇催化剂Cu 4 @Mo 2 C(001)。在这项研究中,使用密度泛函理论(DFT)计算来探索RWGS反应在Cu @ Mo 2 C(001)和Cu 4 @Mo 2 C(001)表面的反应机理。H 2的解离吸附在这两个表面上的碳原子是无障碍的并且高度放热,这对RWGS反应是有利的。重要的是,已经讨论了三种可能的机制-COOH机制,HCOO机制和氧化还原机制。结果表明,氧化还原机制是Cu @ Mo 2 C(001)和Cu 4 @Mo 2 C(001)表面之间最可行的途径。通过比较两个表面上氧化还原机理的限速步骤的激活势垒,结果表明限速步骤对Cu 4 @Mo 2的激活势垒C(001)表面较小,这更有利于RWGS反应的进行。因此,引入非贵金属铜的策略可能是设计高效催化剂的一种有前途的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号