当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cation-π Interactions between Methylated Ammonium Groups and Tryptophan in the CHARMM36 Additive Force Field.
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2018-12-28 , DOI: 10.1021/acs.jctc.8b00839 Hanif M Khan 1, 2 , Alexander D MacKerell 3 , Nathalie Reuter 2, 4
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2018-12-28 , DOI: 10.1021/acs.jctc.8b00839 Hanif M Khan 1, 2 , Alexander D MacKerell 3 , Nathalie Reuter 2, 4
Affiliation
|
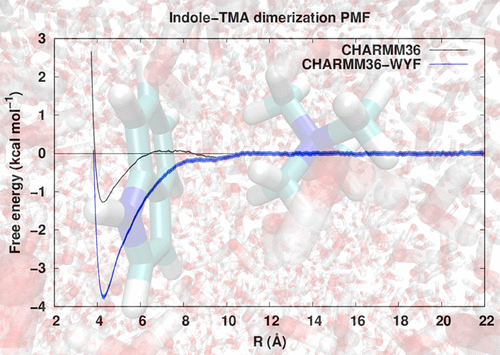
Cation-π interactions between tryptophan and choline or trimethylated lysines are vital for many biological processes. The performance of the additive CHARMM36 force field against target quantum mechanical data is shown to reproduce QM equilibrium geometries but required modified Lennard-Jones potentials to accurately reproduce the QM interaction energies. The modified parameter set allows accurate modeling, including free energies, of cation-π indole-choline and indole-trimethylated lysines interactions relevant for protein-ligand, protein-membrane, and protein-protein interfaces.
中文翻译:

CHARMM36加和力场中甲基化铵基团与色氨酸之间的阳离子-π相互作用。
色氨酸与胆碱或三甲基赖氨酸之间的阳离子-π相互作用对于许多生物过程至关重要。显示了针对目标量子力学数据的加性CHARMM36力场的性能可重现QM平衡几何,但需要修改的Lennard-Jones势能才能准确重现QM相互作用能。修改后的参数集允许对与蛋白质-配体,蛋白质-膜和蛋白质-蛋白质界面相关的阳离子-π吲哚-胆碱和吲哚-三甲基化赖氨酸相互作用进行精确建模,包括自由能。
更新日期:2018-12-18
中文翻译:

CHARMM36加和力场中甲基化铵基团与色氨酸之间的阳离子-π相互作用。
色氨酸与胆碱或三甲基赖氨酸之间的阳离子-π相互作用对于许多生物过程至关重要。显示了针对目标量子力学数据的加性CHARMM36力场的性能可重现QM平衡几何,但需要修改的Lennard-Jones势能才能准确重现QM相互作用能。修改后的参数集允许对与蛋白质-配体,蛋白质-膜和蛋白质-蛋白质界面相关的阳离子-π吲哚-胆碱和吲哚-三甲基化赖氨酸相互作用进行精确建模,包括自由能。































 京公网安备 11010802027423号
京公网安备 11010802027423号