当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Dehydrogenation of Partially Deuterated Ammonia Borane NH3BD3 Being Heating to 200 °C
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-12-19 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02721 Jean-Fabien Petit 1 , Umit B. Demirci 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-12-19 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02721 Jean-Fabien Petit 1 , Umit B. Demirci 1
Affiliation
|
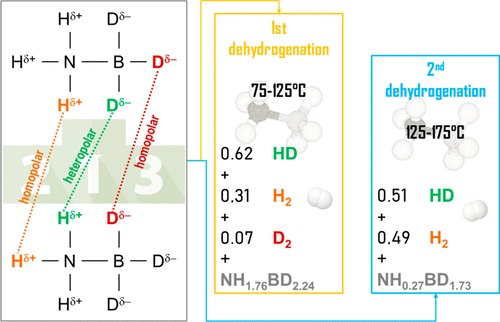
Ammonia borane NH3BH3 is a well-known thermolytic hydrogen storage material. However, the mechanism of dehydrogenation under heating is very complex. In this context, we have studied the thermolytic dehydrocoupling of solid, partially deuterated, ammonia borane NH3BD3 up to 200 °C, that is, up to the release of the second equivalent of hydrogen, by using thermogravimetric analysis, differential scanning calorimetry, and mass spectrometry. Our results show that the process, resulting in the release of hydrogen (i.e., HD), is mainly driven by heteropolar hydrogen–deuterium interactions (N–Hδ+···δ−D–B). Homopolar dihydrogen interactions (N–Hδ+···δ+H–N) appreciably contribute to hydrogen (i.e., H2) release. In contrast, the contribution of homopolar dideuterium interactions (B–Dδ−···δ−D–B) is negligible. In summary, this work sheds new light on the mechanism governing the release of hydrogen from ammonia borane under thermolytic conditions.
中文翻译:

加热至200°C的部分氘代氨硼烷NH 3 BD 3脱氢的机理分析
氨硼烷NH 3 BH 3是众所周知的热解氢存储材料。但是,加热下的脱氢机理非常复杂。在此背景下,我们使用热重分析,差示扫描量热法研究了高达200°C(即直至释放出第二当量的氢)的固态,部分氘代的氨硼烷NH 3 BD 3的热解脱氢反应。和质谱。我们的结果表明,该过程导致氢(即HD)的释放,主要是由杂极性氢-氘相互作用(N– Hδ + ··· δ -D–B)驱动的。同极性二氢相互作用(N– Hδ+ ···δ+ H–N)明显促进了氢(即H 2)的释放。相反,同极性二氘相互作用(B– Dδ- ·· δ -D–B)的贡献可忽略不计。总之,这项工作为控制在热解条件下氨硼烷中释放氢的机理提供了新的思路。
更新日期:2018-12-19
中文翻译:

加热至200°C的部分氘代氨硼烷NH 3 BD 3脱氢的机理分析
氨硼烷NH 3 BH 3是众所周知的热解氢存储材料。但是,加热下的脱氢机理非常复杂。在此背景下,我们使用热重分析,差示扫描量热法研究了高达200°C(即直至释放出第二当量的氢)的固态,部分氘代的氨硼烷NH 3 BD 3的热解脱氢反应。和质谱。我们的结果表明,该过程导致氢(即HD)的释放,主要是由杂极性氢-氘相互作用(N– Hδ + ··· δ -D–B)驱动的。同极性二氢相互作用(N– Hδ+ ···δ+ H–N)明显促进了氢(即H 2)的释放。相反,同极性二氘相互作用(B– Dδ- ·· δ -D–B)的贡献可忽略不计。总之,这项工作为控制在热解条件下氨硼烷中释放氢的机理提供了新的思路。






































 京公网安备 11010802027423号
京公网安备 11010802027423号