Nature Catalysis ( IF 42.8 ) Pub Date : 2018-12-17 , DOI: 10.1038/s41929-018-0198-y Xiaoqiang Huang , Qi Zhang , Jiahui Lin , Klaus Harms , Eric Meggers
|
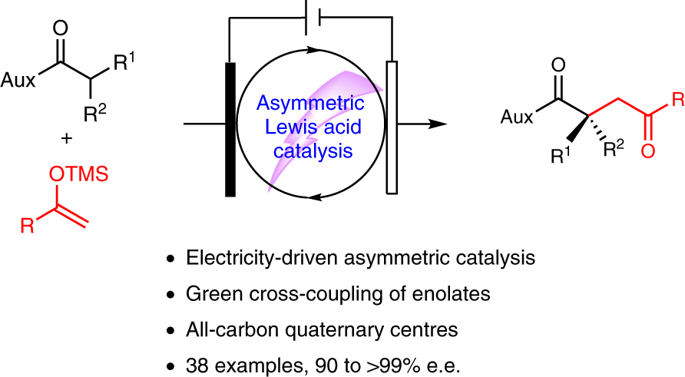
Catalytic asymmetric electrosynthesis combines the unique features of an electrochemical addition or removal of electrons with the catalytic asymmetric synthesis of enantioenriched molecules. However, identifying suitable catalysts that are compatible with electrochemical conditions and provide a high stereocontrol is a formidable challenge. Here we introduce a versatile electricity-driven chiral Lewis acid catalysis for the oxidative cross-coupling of 2-acyl imidazoles with silyl enol ethers. Powered by an electric current, this work provides a sustainable avenue to synthetically useful non-racemic 1,4-dicarbonyls, which include products that bear all-carbon quaternary stereocentres. A chiral-at-metal rhodium catalyst activates a substrate towards anodic oxidation by raising the highest occupied molecular orbital on enolate formation, which enables mild redox conditions, high chemo- and enantioselectivities (up to >99% enantiomeric excess) and a broad substrate scope. This work demonstrates the potential of combining asymmetric Lewis acid catalysis with electrochemistry and we anticipate that it will spur the further development of catalytic asymmetric electrosynthesis.
中文翻译:

电驱动的不对称路易斯酸催化
催化不对称电合成将对电子进行电化学加成或去除的独特功能与对映体富集分子的催化不对称合成相结合。然而,鉴定与电化学条件相容并提供高立体控制的合适催化剂是艰巨的挑战。在这里,我们介绍了一种通用的电力驱动手性路易斯酸催化方法,用于将2-酰基咪唑与甲硅烷基烯醇醚进行氧化交叉偶联。在电流的作用下,这项工作为合成有用的非外消旋1,4-二羰基化合物(包括带有全碳四元立体中心的产品)提供了可持续的途径。手性金属铑催化剂通过增加烯醇盐形成时占据的最高分子轨道,使基质向阳极氧化活化。可实现温和的氧化还原条件,高的化学和对映体选择性(高达> 99%的对映体过量)和广泛的底物范围。这项工作表明不对称路易斯酸催化与电化学相结合的潜力,我们预计它将刺激催化不对称电化学合成的进一步发展。































 京公网安备 11010802027423号
京公网安备 11010802027423号