当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fabrication of poly(β-cyclodextrin)-conjugated magnetic graphene oxide by surface-initiated RAFT polymerization for synergetic adsorption of heavy metal ions and organic pollutants
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-12-18 00:00:00 , DOI: 10.1039/c8ta09250h Jingjing Wang 1, 2, 3, 4 , Wenhui Zhang 2, 3, 4, 5 , Jun Wei 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-12-18 00:00:00 , DOI: 10.1039/c8ta09250h Jingjing Wang 1, 2, 3, 4 , Wenhui Zhang 2, 3, 4, 5 , Jun Wei 1, 2, 3, 4
Affiliation
|
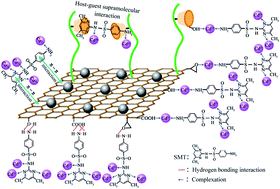
In order to realize synergetic adsorption of heavy metal ions and organic pollutants, poly(β-cyclodextrin)-conjugated magnetic graphene oxide (MGO@poly(β-CD)) was designed and fabricated. The MGO@poly(β-CD) was characterized by Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy, transmission electron microscopy, X-ray diffraction and vibrating sample magnetometry, respectively. Results indicated that the MGO@poly(β-CD) was successfully prepared and the adsorbent could be separated easily once an external magnetic field was applied. The adsorption behaviors of Cd2+ and sulfamethazine (SMT) by MGO@poly(β-CD) in the single and binary systems were investigated. The adsorption amounts of both Cd2+ and SMT were pH-dependent, and the increase in the ionic strength of solutions resulted in significant decrease of their adsorption amounts. The adsorption of Cd2+ and SMT on MGO@poly(β-CD) was achieved by different mechanisms, respectively. The sorption of Cd2+ was related to complexation and electrostatic interactions, while the removal of SMT was realized by hydrogen bonding, host–guest supramolecular and π–π interactions. The synergetic adsorption of Cd2+ and SMT in the binary systems was attributed to interactions between loaded Cd2+ (SMT) and free SMT (Cd2+) in solutions. These results provided valuable information for designing novel adsorbents that can be used for synergetic adsorption of heavy metal ions and organic pollutants.
中文翻译:

表面引发的RAFT聚合制备聚(β-环糊精)共轭磁性氧化石墨烯,用于重金属离子和有机污染物的协同吸附
为了实现重金属离子与有机污染物的协同吸附,设计并制备了聚(β-环糊精)-共轭磁性氧化石墨烯(MGO @ poly(β-CD))。MGO @ poly(β-CD)分别用傅里叶变换红外光谱,X射线光电子能谱,扫描电子显微镜,透射电子显微镜,X射线衍射和振动样品磁强法进行了表征。结果表明,成功制备了MGO @ poly(β-CD),一旦施加外部磁场,吸附剂就很容易分离。研究了MGO @ poly(β-CD)在单和二元体系中对Cd 2+和磺胺二甲嘧啶(SMT)的吸附行为。Cd 2+的吸附量SMT和SMT是pH依赖性的,溶液离子强度的增加导致它们的吸附量显着降低。CGO 2+和SMT在MGO @ poly(β-CD)上的吸附机理是不同的。Cd 2+的吸附与络合和静电相互作用有关,而SMT的去除是通过氢键,主体-客体超分子和π-π相互作用实现的。Cd 2+和SMT在二元系统中的协同吸附归因于负载的Cd 2+(SMT)与游离SMT(Cd 2+)的解决方案。这些结果为设计可用于重金属离子和有机污染物的协同吸附的新型吸附剂提供了有价值的信息。
更新日期:2018-12-18
中文翻译:

表面引发的RAFT聚合制备聚(β-环糊精)共轭磁性氧化石墨烯,用于重金属离子和有机污染物的协同吸附
为了实现重金属离子与有机污染物的协同吸附,设计并制备了聚(β-环糊精)-共轭磁性氧化石墨烯(MGO @ poly(β-CD))。MGO @ poly(β-CD)分别用傅里叶变换红外光谱,X射线光电子能谱,扫描电子显微镜,透射电子显微镜,X射线衍射和振动样品磁强法进行了表征。结果表明,成功制备了MGO @ poly(β-CD),一旦施加外部磁场,吸附剂就很容易分离。研究了MGO @ poly(β-CD)在单和二元体系中对Cd 2+和磺胺二甲嘧啶(SMT)的吸附行为。Cd 2+的吸附量SMT和SMT是pH依赖性的,溶液离子强度的增加导致它们的吸附量显着降低。CGO 2+和SMT在MGO @ poly(β-CD)上的吸附机理是不同的。Cd 2+的吸附与络合和静电相互作用有关,而SMT的去除是通过氢键,主体-客体超分子和π-π相互作用实现的。Cd 2+和SMT在二元系统中的协同吸附归因于负载的Cd 2+(SMT)与游离SMT(Cd 2+)的解决方案。这些结果为设计可用于重金属离子和有机污染物的协同吸附的新型吸附剂提供了有价值的信息。































 京公网安备 11010802027423号
京公网安备 11010802027423号