Nature Chemistry ( IF 19.2 ) Pub Date : 2018-12-17 , DOI: 10.1038/s41557-018-0175-8
Jinpeng Zhao , Takeshi Nanjo , Emilio C. de Lucca , M. Christina White
|
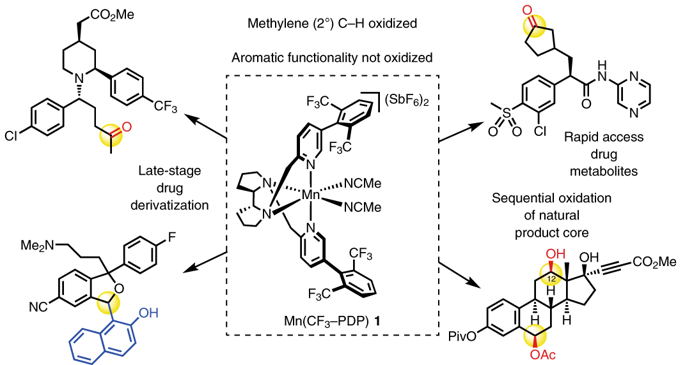
Despite significant progress in the development of site-selective aliphatic C–H oxidations over the past decade, the ability to oxidize strong methylene C–H bonds in the presence of more oxidatively labile aromatic functionalities remains a major unsolved problem. Such chemoselective reactivity is highly desirable for enabling late-stage oxidative derivatizations of pharmaceuticals and medicinally important natural products that often contain such functionality. Here, we report a simple manganese small-molecule catalyst Mn(CF3–PDP) system that achieves such chemoselectivity via an unexpected synergy of catalyst design and acid additive. Preparative remote methylene oxidation is obtained in 50 aromatic compounds housing medicinally relevant halogen, oxygen, heterocyclic and biaryl moieties. Late-stage methylene oxidation is demonstrated on four drug scaffolds, including the ethinylestradiol scaffold where other non-directed C–H oxidants that tolerate aromatic groups effect oxidation at only activated tertiary benzylic sites. Rapid generation of a known metabolite (piragliatin) from an advanced intermediate is demonstrated.
中文翻译:

芳香族分子中的化学选择性亚甲基氧化
尽管在过去十年中,位点选择性脂肪族C–H氧化的发展取得了重大进展,但是在存在更多易氧化的芳族官能团的情况下,能够氧化亚甲基C–H强键的能力仍然是一个主要未解决的问题。这种化学选择性反应性对于使通常包含这种功能性的药物和重要的天然药物能够进行后期的氧化衍生化是非常需要的。在这里,我们报告了一种简单的锰小分子催化剂Mn(CF 3-PDP)系统通过催化剂设计和酸添加剂的意外协同作用实现了这种化学选择性。在50种芳香族化合物中可进行制备型亚甲基远距离氧化,这些芳香族化合物具有可药用的卤素,氧,杂环和联芳基部分。在四种药物支架上均显示了晚期亚甲基氧化,包括乙炔雌二醇支架,在该支架上,其他可耐受芳香族基团的非定向CH氧化剂仅在活化的叔苄基位上进行氧化。证明了从高级中间体快速生成已知代谢物(吡拉格列汀)的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号