当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differentiation of primate primordial germ cell-like cells following transplantation into the adult gonadal niche.
Nature Communications ( IF 14.7 ) Pub Date : 2018-12-17 , DOI: 10.1038/s41467-018-07740-7 Enrique Sosa 1 , Di Chen 1 , Ernesto J Rojas 1 , Jon D Hennebold 2, 3 , Karen A Peters 4 , Zhuang Wu 5 , Truong N Lam 5 , Jennifer M Mitchell 6 , Meena Sukhwani 4 , Ramesh C Tailor 7 , Marvin L Meistrich 5 , Kyle E Orwig 4 , Gunapala Shetty 5 , Amander T Clark 1
Nature Communications ( IF 14.7 ) Pub Date : 2018-12-17 , DOI: 10.1038/s41467-018-07740-7 Enrique Sosa 1 , Di Chen 1 , Ernesto J Rojas 1 , Jon D Hennebold 2, 3 , Karen A Peters 4 , Zhuang Wu 5 , Truong N Lam 5 , Jennifer M Mitchell 6 , Meena Sukhwani 4 , Ramesh C Tailor 7 , Marvin L Meistrich 5 , Kyle E Orwig 4 , Gunapala Shetty 5 , Amander T Clark 1
Affiliation
|
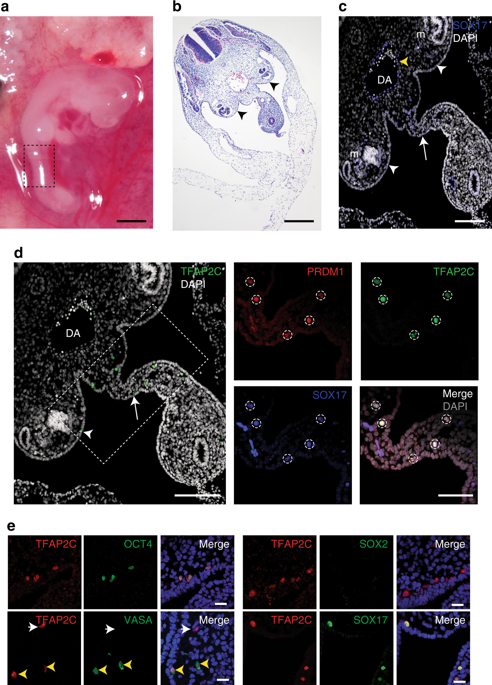
A major challenge in stem cell differentiation is the availability of bioassays to prove cell types generated in vitro are equivalent to cells in vivo. In the mouse, differentiation of primordial germ cell-like cells (PGCLCs) from pluripotent cells was validated by transplantation, leading to the generation of spermatogenesis and to the birth of offspring. Here we report the use of xenotransplantation (monkey to mouse) and homologous transplantation (monkey to monkey) to validate our in vitro protocol for differentiating male rhesus (r) macaque PGCLCs (rPGCLCs) from induced pluripotent stem cells (riPSCs). Specifically, transplantation of aggregates containing rPGCLCs into mouse and nonhuman primate testicles overcomes a major bottleneck in rPGCLC differentiation. These findings suggest that immature rPGCLCs once transplanted into an adult gonadal niche commit to differentiate towards late rPGCs that initiate epigenetic reprogramming but do not complete the conversion into ENO2-positive spermatogonia.
中文翻译:

植入成年性腺利基后,灵长类动物原始生殖细胞样细胞的分化。
干细胞分化的主要挑战是生物测定的有效性,以证明体外产生的细胞类型等同于体内细胞。在小鼠中,通过移植验证了从多能细胞分化出的原始生殖细胞样细胞(PGCLC),从而导致了精子的生成和后代的诞生。在这里,我们报告了异种移植(猴子到小鼠)和同源移植(猴子到猴子)的使用,以验证我们的体外方案,以区分雄性恒河猴(r)猕猴PGCLC(rPGCLC)与诱导多能干细胞(riPSC)。具体而言,将包含rPGCLC的聚集体移植到小鼠和非人类灵长类动物的睾丸中,克服了rPGCLC分化的主要瓶颈。
更新日期:2018-12-17
中文翻译:

植入成年性腺利基后,灵长类动物原始生殖细胞样细胞的分化。
干细胞分化的主要挑战是生物测定的有效性,以证明体外产生的细胞类型等同于体内细胞。在小鼠中,通过移植验证了从多能细胞分化出的原始生殖细胞样细胞(PGCLC),从而导致了精子的生成和后代的诞生。在这里,我们报告了异种移植(猴子到小鼠)和同源移植(猴子到猴子)的使用,以验证我们的体外方案,以区分雄性恒河猴(r)猕猴PGCLC(rPGCLC)与诱导多能干细胞(riPSC)。具体而言,将包含rPGCLC的聚集体移植到小鼠和非人类灵长类动物的睾丸中,克服了rPGCLC分化的主要瓶颈。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号