当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Alkylation of Ketone Enolates: Synthesis of Monoselective Linear Ketones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-12-14 00:00:00 , DOI: 10.1021/acs.joc.8b02609
Jagadish Das 1 , Mari Vellakkaran 1 , Debasis Banerjee 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-12-14 00:00:00 , DOI: 10.1021/acs.joc.8b02609
Jagadish Das 1 , Mari Vellakkaran 1 , Debasis Banerjee 1
Affiliation
|
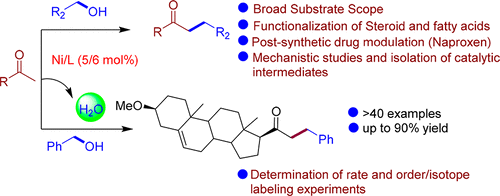
Herein we have developed a Ni-catalyzed protocol for the synthesis of linear ketones. Aryl, alkyl, and heteroaryl ketones as well as alcohols yielded the monoselective ketones in up to 90% yield. The catalytic protocol was successfully applied in to a gram-scale synthesis. For a practical utility, applications of a steroid derivative, oleyl alcohol, and naproxen alcohol were employed. Preliminary catalytic investigations involving the isolation of a Ni intermediate and defined Ni–H species as well as a series of deuterium-labeling experiments were performed.
中文翻译:

镍催化的酮醇酸酯的烷基化:单选择性线性酮的合成
本文中,我们已经开发了镍催化的线性酮合成方案。芳基,烷基和杂芳基酮以及醇类以高达90%的产率生成单选择性酮。催化方案已成功应用于克级合成。为了实用,使用了类固醇衍生物,油醇和萘普生醇的应用。进行了初步催化研究,包括分离镍中间体和确定的Ni–H物种,以及一系列氘标记实验。
更新日期:2018-12-14
中文翻译:

镍催化的酮醇酸酯的烷基化:单选择性线性酮的合成
本文中,我们已经开发了镍催化的线性酮合成方案。芳基,烷基和杂芳基酮以及醇类以高达90%的产率生成单选择性酮。催化方案已成功应用于克级合成。为了实用,使用了类固醇衍生物,油醇和萘普生醇的应用。进行了初步催化研究,包括分离镍中间体和确定的Ni–H物种,以及一系列氘标记实验。

































 京公网安备 11010802027423号
京公网安备 11010802027423号