当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cesium Oleate Precursor Preparation for Lead Halide Perovskite Nanocrystal Synthesis: The Influence of Excess Oleic Acid on Achieving Solubility, Conversion, and Reproducibility
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-12-13 00:00:00 , DOI: 10.1021/acs.chemmater.8b04876 Chang Lu 1 , Marcus W. Wright 1 , Xiao Ma 1 , Hui Li 1 , Dominique S. Itanze 1 , Jake A. Carter 1 , Corey A. Hewitt 2 , George L. Donati 1 , David L. Carroll 2 , Pamela M. Lundin 3 , Scott M. Geyer 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-12-13 00:00:00 , DOI: 10.1021/acs.chemmater.8b04876 Chang Lu 1 , Marcus W. Wright 1 , Xiao Ma 1 , Hui Li 1 , Dominique S. Itanze 1 , Jake A. Carter 1 , Corey A. Hewitt 2 , George L. Donati 1 , David L. Carroll 2 , Pamela M. Lundin 3 , Scott M. Geyer 1
Affiliation
|
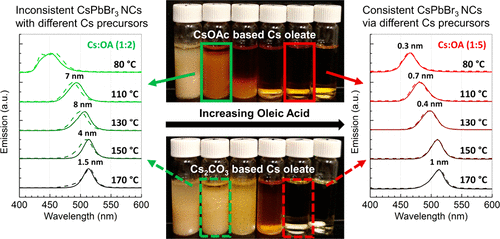
In the colloidal synthesis of inorganic perovskite materials, cesium oleate (CsOL) is the most commonly used Cs precursor. Yet, despite its ubiquitous use in literature, CsOL has been observed to be insoluble at room temperature and leads to surprisingly inconsistent results in CsPbX3 nanocrystal synthesis, depending on the Cs salt from which the precursor is derived. We show that under the conditions used in most reports, the amount of oleic acid (OA) added, while stoichiometrically sufficient, still leads to incomplete conversion of the Cs salts to CsOL. This results in a mixture of Cs sources being present during the reaction, causing decreased homogeneity and reproducibility. When a 1:5 Cs:OA ratio is used, complete conversion is readily obtained, even under mild conditions, resulting in a precursor solution that is soluble at room temperature and yields identical synthetic results, regardless of the initial Cs source. Furthermore, 1H nuclear magnetic resonance (NMR) of solutions prepared using varying Cs:OA ratios shows that the maximum ratio of Cs:OA obtainable in solution is 1:5, with any excess Cs present in the precipitate. We believe the use of a soluble, fully converted CsOL reagent will improve reproducibility for Cs-based perovskite synthesis and directly benefit synthetic methods based on microfluidics.
中文翻译:

卤化钙钛矿纳米晶合成的油酸铯前体制备:过量的油酸对溶解度,转化率和重现性的影响
在无机钙钛矿材料的胶体合成中,油酸铯(CsOL)是最常用的Cs前体。然而,尽管在文献中普遍使用了CsOL,但已观察到CsOL在室温下不溶,并导致CsPbX 3的结果出乎意料地不一致纳米晶体的合成,取决于前体衍生自的Cs盐。我们显示,在大多数报告中使用的条件下,添加的油酸(OA)数量虽然在化学计量上足够,但仍会导致Cs盐向CsOL的不完全转化。这导致反应过程中存在混合的Cs源,导致均一性和重现性降低。当使用1:5 Cs:OA比率时,即使在温和条件下也很容易获得完全转化,从而得到前体溶液,该溶液在室温下可溶,并且无论初始Cs来源如何,其合成结果均相同。此外,1使用变化的Cs:OA比例制备的溶液的H核磁共振(NMR)表明,溶液中可获得的Cs:OA的最大比例为1:5,沉淀物中存在任何过量的Cs。我们相信使用可溶的,完全转化的CsOL试剂将提高基于Cs的钙钛矿合成的可重复性,并直接有益于基于微流体的合成方法。
更新日期:2018-12-13
中文翻译:

卤化钙钛矿纳米晶合成的油酸铯前体制备:过量的油酸对溶解度,转化率和重现性的影响
在无机钙钛矿材料的胶体合成中,油酸铯(CsOL)是最常用的Cs前体。然而,尽管在文献中普遍使用了CsOL,但已观察到CsOL在室温下不溶,并导致CsPbX 3的结果出乎意料地不一致纳米晶体的合成,取决于前体衍生自的Cs盐。我们显示,在大多数报告中使用的条件下,添加的油酸(OA)数量虽然在化学计量上足够,但仍会导致Cs盐向CsOL的不完全转化。这导致反应过程中存在混合的Cs源,导致均一性和重现性降低。当使用1:5 Cs:OA比率时,即使在温和条件下也很容易获得完全转化,从而得到前体溶液,该溶液在室温下可溶,并且无论初始Cs来源如何,其合成结果均相同。此外,1使用变化的Cs:OA比例制备的溶液的H核磁共振(NMR)表明,溶液中可获得的Cs:OA的最大比例为1:5,沉淀物中存在任何过量的Cs。我们相信使用可溶的,完全转化的CsOL试剂将提高基于Cs的钙钛矿合成的可重复性,并直接有益于基于微流体的合成方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号