当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral‐at‐Ruthenium Catalyst with Sterically Demanding Furo[3,2‐b]pyridine Ligands
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2018-12-27 , DOI: 10.1002/ejic.201801362 Tianjiao Cui 1 , Jie Qin 1 , Klaus Harms 1 , Eric Meggers 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2018-12-27 , DOI: 10.1002/ejic.201801362 Tianjiao Cui 1 , Jie Qin 1 , Klaus Harms 1 , Eric Meggers 1
Affiliation
|
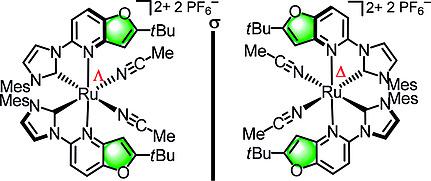
A sterically demanding derivative of a previously introduced chiral‐at‐metal ruthenium(II) catalyst scaffold is introduced. It is composed of two bidentate furo[3,2‐b]pyridyl functionalized N‐heterocyclic carbene ligands. Their cis‐coordination generates helical chirality and a stereogenic ruthenium center. Two additional labile acetonitriles compose the catalytic site which is highly shielded by two 2‐(tert‐butyl)furo[3,2‐b]pyridine moieties. The synthesis of the non‐racemic ruthenium catalyst and its catalytic properties for the enantioselective alkynylation of 2,2,2‐trifluoroacetophenone and pentafluorobenzaldehyde are reported and compared with sterically less demanding derivatives.
中文翻译:

具有空间需求的呋喃[3,2-b]吡啶配体的手性钌催化剂
引入了先前引入的手性金属钌(II)催化剂支架的空间要求较高的衍生物。它由两个二齿呋喃并[3,2- b ]吡啶基官能化的N-杂环卡宾配体组成。它们的顺式配位产生了螺旋手性和一个立体的钌中心。另外两个不稳定的乙腈组成了催化位点,该位点被两个2-(叔丁基)呋喃[3,2- b ]吡啶部分高度屏蔽。报告了非外消旋钌催化剂的合成及其对2,2,2-三氟苯乙酮和五氟苯甲醛的对映选择性炔基化反应的催化性能,并将其与空间上要求不高的衍生物进行了比较。
更新日期:2018-12-27
中文翻译:

具有空间需求的呋喃[3,2-b]吡啶配体的手性钌催化剂
引入了先前引入的手性金属钌(II)催化剂支架的空间要求较高的衍生物。它由两个二齿呋喃并[3,2- b ]吡啶基官能化的N-杂环卡宾配体组成。它们的顺式配位产生了螺旋手性和一个立体的钌中心。另外两个不稳定的乙腈组成了催化位点,该位点被两个2-(叔丁基)呋喃[3,2- b ]吡啶部分高度屏蔽。报告了非外消旋钌催化剂的合成及其对2,2,2-三氟苯乙酮和五氟苯甲醛的对映选择性炔基化反应的催化性能,并将其与空间上要求不高的衍生物进行了比较。































 京公网安备 11010802027423号
京公网安备 11010802027423号