当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brønsted–Lowry Acid Strength of Metal Hydride and Dihydrogen Complexes
Chemical Reviews ( IF 51.4 ) Pub Date : 2016-03-10 00:00:00 , DOI: 10.1021/acs.chemrev.5b00695 Robert H. Morris 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2016-03-10 00:00:00 , DOI: 10.1021/acs.chemrev.5b00695 Robert H. Morris 1
Affiliation
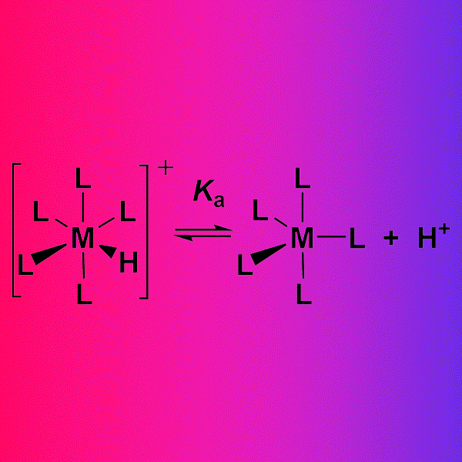
|
Transition metal hydride complexes are usually amphoteric, not only acting as hydride donors, but also as Brønsted–Lowry acids. A simple additive ligand acidity constant equation (LAC for short) allows the estimation of the acid dissociation constant KaLAC of diamagnetic transition metal hydride and dihydrogen complexes. It is remarkably successful in systematizing diverse reports of over 450 reactions of acids with metal complexes and bases with metal hydrides and dihydrogen complexes, including catalytic cycles where these reactions are proposed or observed. There are links between pKaLAC and pKaTHF, pKaDCM, pKaMeCN for neutral and cationic acids. For the groups from chromium to nickel, tables are provided that order the acidity of metal hydride and dihydrogen complexes from most acidic (pKaLAC −18) to least acidic (pKaLAC 50). Figures are constructed showing metal acids above the solvent pKa scales and organic acids below to summarize a large amount of information. Acid–base features are analyzed for catalysts from chromium to gold for ionic hydrogenations, bifunctional catalysts for hydrogen oxidation and evolution electrocatalysis, H/D exchange, olefin hydrogenation and isomerization, hydrogenation of ketones, aldehydes, imines, and carbon dioxide, hydrogenases and their model complexes, and palladium catalysts with hydride intermediates.
中文翻译:

金属氢化物和二氢配合物的布朗斯台德-低酸强度
过渡金属氢化物络合物通常是两性的,不仅充当氢化物供体,而且还充当布朗斯台德-洛里酸。一个简单的加成配体酸度常数方程式(简称LAC)可以估算出反磁性过渡金属氢化物和二氢配合物的酸解离常数K a LAC。在将酸与金属配合物和碱与金属氢化物和二氢配合物进行的450多种反应的系统化报告中取得显著成功,包括提出或观察到这些反应的催化循环。在p K a LAC和p K a THF,p K a DCM,p K之间存在联系用于中性和阳离子酸的MeCN。对于从铬到镍的基团,提供了表,其将金属氢化物和二氢配合物的酸度从最酸性(p K a LAC -18)变为最不酸性(p K a LAC 50)。绘制的图显示溶剂p K a以上的金属酸下面以比例尺和有机酸来总结大量信息。分析了酸碱特性,包括从铬到金的离子加氢催化剂,用于氢氧化和析出电催化,H / D交换,烯烃加氢和异构化,酮,醛,亚胺和二氧化碳加氢的双功能催化剂,加氢酶及其衍生物模型配合物,以及带有氢化物中间体的钯催化剂。
更新日期:2016-03-10
中文翻译:

金属氢化物和二氢配合物的布朗斯台德-低酸强度
过渡金属氢化物络合物通常是两性的,不仅充当氢化物供体,而且还充当布朗斯台德-洛里酸。一个简单的加成配体酸度常数方程式(简称LAC)可以估算出反磁性过渡金属氢化物和二氢配合物的酸解离常数K a LAC。在将酸与金属配合物和碱与金属氢化物和二氢配合物进行的450多种反应的系统化报告中取得显著成功,包括提出或观察到这些反应的催化循环。在p K a LAC和p K a THF,p K a DCM,p K之间存在联系用于中性和阳离子酸的MeCN。对于从铬到镍的基团,提供了表,其将金属氢化物和二氢配合物的酸度从最酸性(p K a LAC -18)变为最不酸性(p K a LAC 50)。绘制的图显示溶剂p K a以上的金属酸下面以比例尺和有机酸来总结大量信息。分析了酸碱特性,包括从铬到金的离子加氢催化剂,用于氢氧化和析出电催化,H / D交换,烯烃加氢和异构化,酮,醛,亚胺和二氧化碳加氢的双功能催化剂,加氢酶及其衍生物模型配合物,以及带有氢化物中间体的钯催化剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号