当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In vitro reconstitution of the biosynthetic pathway of 3-hydroxypicolinic acid
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-12-10 , DOI: 10.1039/c8ob02972e Xuan Yun 1, 2, 3, 4, 5 , Qian Zhang 1, 2, 3, 4, 5 , Meinan Lv 1, 2, 3, 4, 5 , Hai Deng 6, 7, 8, 9, 10 , Zixin Deng 1, 2, 3, 4, 5 , Yi Yu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-12-10 , DOI: 10.1039/c8ob02972e Xuan Yun 1, 2, 3, 4, 5 , Qian Zhang 1, 2, 3, 4, 5 , Meinan Lv 1, 2, 3, 4, 5 , Hai Deng 6, 7, 8, 9, 10 , Zixin Deng 1, 2, 3, 4, 5 , Yi Yu 1, 2, 3, 4, 5
Affiliation
|
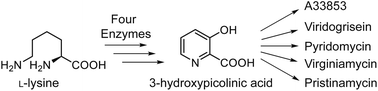
3-Hydroxypicolinic acid (3-HPA) is an important pyridine building block of bacterial secondary metabolites. Although the main biosynthetic pathways of these metabolites have been identified and well characterized, the enzymatic mechanism underlying the biosynthesis of 3-HPA has yet to be elucidated. In this work, we successfully reconstituted the complete biosynthetic pathway of 3-HPA in vitro. We showed that an L-lysine 2-aminotransferase, a two-component monooxygenase, and a FAD-dependent dehydrogenase are required to convert L-lysine to 3-HPA. We further demonstrated that 3-HPA does not derive from the direct hydroxylation of the picolinic acid at C-3, but from a successive process of C-3 hydroxylation of the piperideine-2-carboxylic acid and tautomerization of the produced 3-hydroxyl dihydropicolinic acid. Therefore, this study unveils the unusual assembly logic of 3-HPA and sheds light on the potential of engineering the 3-HPA pathway for generating novel pyridine-based building blocks.
中文翻译:

3-羟基吡啶甲酸的生物合成途径的体外重建
3-羟基吡啶甲酸(3-HPA)是细菌次级代谢产物的重要吡啶构建基。尽管已鉴定出这些代谢物的主要生物合成途径并对其进行了很好的表征,但尚未阐明3-HPA的生物合成基础的酶促机理。在这项工作中,我们成功地重建了体外3-HPA的完整生物合成途径。我们表明,需要L-赖氨酸2-氨基转移酶,两组分单加氧酶和FAD依赖的脱氢酶来转化L-赖氨酸为3-HPA。我们进一步证明3-HPA并非源自吡啶甲酸在C-3处的直接羟基化,而是源自哌啶子基-2-羧酸的C-3羟基化和所产生的3-羟基二氢吡啶甲酸互变异构的连续过程酸。因此,这项研究揭示了3-HPA的非同寻常的装配逻辑,并阐明了工程化3-HPA途径产生新型吡啶基构件的潜力。
更新日期:2019-01-17
中文翻译:

3-羟基吡啶甲酸的生物合成途径的体外重建
3-羟基吡啶甲酸(3-HPA)是细菌次级代谢产物的重要吡啶构建基。尽管已鉴定出这些代谢物的主要生物合成途径并对其进行了很好的表征,但尚未阐明3-HPA的生物合成基础的酶促机理。在这项工作中,我们成功地重建了体外3-HPA的完整生物合成途径。我们表明,需要L-赖氨酸2-氨基转移酶,两组分单加氧酶和FAD依赖的脱氢酶来转化L-赖氨酸为3-HPA。我们进一步证明3-HPA并非源自吡啶甲酸在C-3处的直接羟基化,而是源自哌啶子基-2-羧酸的C-3羟基化和所产生的3-羟基二氢吡啶甲酸互变异构的连续过程酸。因此,这项研究揭示了3-HPA的非同寻常的装配逻辑,并阐明了工程化3-HPA途径产生新型吡啶基构件的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号