当前位置:
X-MOL 学术
›
Commun. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The crystal structure of a tetrahydrofolate-bound dihydrofolate reductase reveals the origin of slow product release.
Communications Biology ( IF 5.2 ) Pub Date : 2018-12-12 , DOI: 10.1038/s42003-018-0236-y Hongnan Cao 1 , Mu Gao 1 , Hongyi Zhou 1 , Jeffrey Skolnick 1
Communications Biology ( IF 5.2 ) Pub Date : 2018-12-12 , DOI: 10.1038/s42003-018-0236-y Hongnan Cao 1 , Mu Gao 1 , Hongyi Zhou 1 , Jeffrey Skolnick 1
Affiliation
|
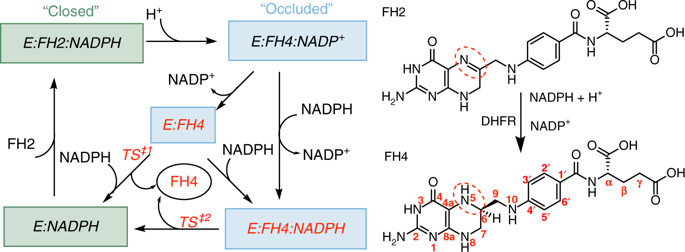
Dihydrofolate reductase (DHFR) catalyzes the stereospecific reduction of 7,8-dihydrofolate (FH2) to (6s)-5,6,7,8-tetrahydrofolate (FH4) via hydride transfer from NADPH. The consensus Escherichia coli DHFR mechanism involves conformational changes between closed and occluded states occurring during the rate-limiting product release step. Although the Protein Data Bank (PDB) contains over 250 DHFR structures, the FH4 complex structure responsible for rate-limiting product release is unknown. We report to our knowledge the first crystal structure of an E. coli. DHFR:FH4 complex at 1.03 Å resolution showing distinct stabilizing interactions absent in FH2 or related (6R)-5,10-dideaza-FH4 complexes. We discover the time course of decay of the co-purified endogenous FH4 during crystal growth, with conversion from FH4 to FH2 occurring in 2-3 days. We also determine another occluded complex structure of E. coli DHFR with a slow-onset nanomolar inhibitor that contrasts with the methotrexate complex, suggesting a plausible strategy for designing DHFR antibiotics by targeting FH4 product conformations.
更新日期:2019-01-26































 京公网安备 11010802027423号
京公网安备 11010802027423号