Nature Chemistry ( IF 19.2 ) Pub Date : 2018-12-10 , DOI: 10.1038/s41557-018-0187-4 Zufeng Guo , Sam Y. Hong , Jingxin Wang , Shahid Rehan , Wukun Liu , Hanjing Peng , Manisha Das , Wei Li , Shridhar Bhat , Brandon Peiffer , Brett R. Ullman , Chung-Ming Tse , Zlatina Tarmakova , Cordelia Schiene-Fischer , Gunter Fischer , Imogen Coe , Ville O. Paavilainen , Zhaoli Sun , Jun O. Liu
|
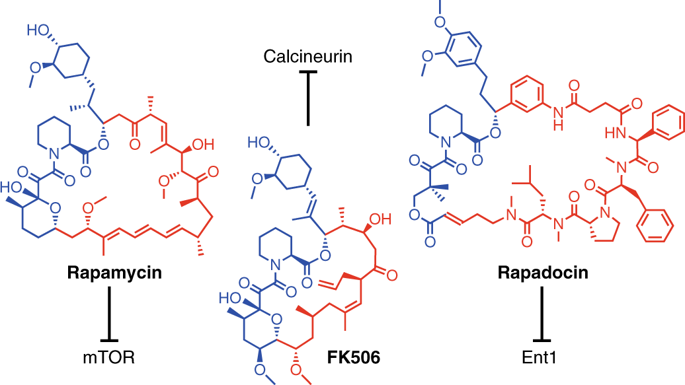
Rapamycin and FK506 are macrocyclic natural products with an extraordinary mode of action, in which they form binary complexes with FK506-binding protein (FKBP) through a shared FKBP-binding domain before forming ternary complexes with their respective targets, mechanistic target of rapamycin (mTOR) and calcineurin, respectively. Inspired by this, we sought to build a rapamycin-like macromolecule library to target new cellular proteins by replacing the effector domain of rapamycin with a combinatorial library of oligopeptides. We developed a robust macrocyclization method using ring-closing metathesis and synthesized a 45,000-compound library of hybrid macrocycles (named rapafucins) using optimized FKBP-binding domains. Screening of the rapafucin library in human cells led to the discovery of rapadocin, an inhibitor of nucleoside uptake. Rapadocin is a potent, isoform-specific and FKBP-dependent inhibitor of the equilibrative nucleoside transporter 1 and is efficacious in an animal model of kidney ischaemia reperfusion injury. Together, these results demonstrate that rapafucins are a new class of chemical probes and drug leads that can expand the repertoire of protein targets well beyond mTOR and calcineurin.
中文翻译:

雷帕霉素启发的大环化合物具有新的靶标特异性
雷帕霉素和FK506是具有非凡作用方式的大环天然产物,其中它们通过共享的FKBP结合域与FK506结合蛋白(FKBP)形成二元复合物,然后与各自的靶标(雷帕霉素的机械靶标)形成三元复合物(mTOR )和钙调神经磷酸酶。受此启发,我们试图通过用寡肽组合文库取代雷帕霉素的效应子域来构建雷帕霉素样大分子文库,以靶向新的细胞蛋白。我们开发了一种使用闭环复分解的稳健的大环化方法,并使用优化的FKBP结合域合成了45,000化合物的混合大环化合物库(命名为rapafucins)。在人细胞中对雷帕霉素蛋白文库的筛选导致了雷帕多星(一种核苷摄取抑制剂)的发现。雷帕多辛是平衡核苷转运蛋白1的有效,同工型特异性和FKBP依赖性抑制剂,在肾缺血再灌注损伤的动物模型中有效。总之,这些结果表明,雷帕福星是一类新型的化学探针和药物引线,可以扩展蛋白质靶标的范围,远远超过mTOR和钙调神经磷酸酶。































 京公网安备 11010802027423号
京公网安备 11010802027423号