当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total enzyme syntheses of napyradiomycins A1 and B1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-12-10 , DOI: 10.1021/jacs.8b10134 Shaun M. K. McKinnie 1 , Zachary D. Miles 1 , Peter A. Jordan 1 , Takayoshi Awakawa 1 , Henry P. Pepper 2 , Lauren A. M. Murray 2 , Jonathan H. George 2 , Bradley S. Moore 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-12-10 , DOI: 10.1021/jacs.8b10134 Shaun M. K. McKinnie 1 , Zachary D. Miles 1 , Peter A. Jordan 1 , Takayoshi Awakawa 1 , Henry P. Pepper 2 , Lauren A. M. Murray 2 , Jonathan H. George 2 , Bradley S. Moore 1, 3
Affiliation
|
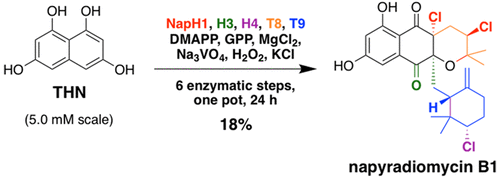
The biosynthetic route to the napyradiomycin family of bacterial meroterpenoids has been fully described 32 years following their original isolation and 11 years after their gene cluster discovery. The antimicrobial and cytotoxic natural products napyradiomycins A1 and B1 are produced using three organic substrates (1,3,6,8-tetrahydroxynaphthalene, dimethylallyl pyrophosphate, and geranyl pyrophosphate), and catalysis via five enzymes: two aromatic prenyltransferases (NapT8 and T9); and three vanadium dependent haloperoxidase (VHPO) homologues (NapH1, H3, and H4). Building upon the previous characterization of NapH1, H3, and T8, we herein describe the initial (NapT9, H1) and final (NapH4) steps required for napyradiomycin construction. This remarkably streamlined biosynthesis highlights the utility of VHPO enzymology in complex natural product generation, as NapH4 efficiently performs a unique chloronium-induced terpenoid cyclization to establish two stereocenters and a new carbon-carbon bond, and dual-acting NapH1 catalyzes chlorination and etherification reactions at two distinct stages of the pathway. Moreover, we employed recombinant napyradiomycin biosynthetic enzymes to chemoenzymatically synthesize milligram quantities in one pot in 1 day. This method represents a viable enantioselective approach to produce complex halogenated metabolites, like napyradiomycin B1, that have yet to be chemically synthesized.
中文翻译:

萘啶霉素 A1 和 B1 的总酶合成
细菌类萜类萘放射霉素家族的生物合成途径在它们最初分离后 32 年和基因簇发现后 11 年已被充分描述。抗菌和细胞毒性天然产物萘啶霉素 A1 和 B1 使用三种有机底物(1,3,6,8-四羟基萘、二甲基烯丙基焦磷酸和焦磷酸香叶基)和五种酶催化生产:两种芳香异戊二烯转移酶(NapT8 和 T9);和三个钒依赖性卤代过氧化物酶 (VHPO) 同系物(NapH1、H3 和 H4)。基于先前对 NapH1、H3 和 T8 的表征,我们在此描述了萘二甲酸构建所需的初始 (NapT9、H1) 和最终 (NapH4) 步骤。这种显着简化的生物合成突出了 VHPO 酶学在复杂天然产物生成中的效用,因为 NapH4 有效地进行了独特的氯铵诱导的萜类环化,以建立两个立体中心和一个新的碳-碳键,而双作用 NapH1 催化氯化和醚化反应在路径的两个不同阶段。此外,我们使用重组萘放射霉素生物合成酶在 1 天内在一锅中化学酶法合成毫克量。这种方法代表了一种可行的对映选择性方法来生产复杂的卤代代谢物,如尚未化学合成的萘二甲酸 B1。双作用 NapH1 在该途径的两个不同阶段催化氯化和醚化反应。此外,我们使用重组萘放射霉素生物合成酶在 1 天内在一锅中化学酶法合成毫克量。这种方法代表了一种可行的对映选择性方法来生产复杂的卤代代谢物,如尚未化学合成的萘二甲酸 B1。双作用 NapH1 在该途径的两个不同阶段催化氯化和醚化反应。此外,我们使用重组萘放射霉素生物合成酶在 1 天内在一锅中化学酶法合成毫克量。这种方法代表了一种可行的对映选择性方法来生产复杂的卤代代谢物,如尚未化学合成的萘二甲酸 B1。
更新日期:2018-12-10
中文翻译:

萘啶霉素 A1 和 B1 的总酶合成
细菌类萜类萘放射霉素家族的生物合成途径在它们最初分离后 32 年和基因簇发现后 11 年已被充分描述。抗菌和细胞毒性天然产物萘啶霉素 A1 和 B1 使用三种有机底物(1,3,6,8-四羟基萘、二甲基烯丙基焦磷酸和焦磷酸香叶基)和五种酶催化生产:两种芳香异戊二烯转移酶(NapT8 和 T9);和三个钒依赖性卤代过氧化物酶 (VHPO) 同系物(NapH1、H3 和 H4)。基于先前对 NapH1、H3 和 T8 的表征,我们在此描述了萘二甲酸构建所需的初始 (NapT9、H1) 和最终 (NapH4) 步骤。这种显着简化的生物合成突出了 VHPO 酶学在复杂天然产物生成中的效用,因为 NapH4 有效地进行了独特的氯铵诱导的萜类环化,以建立两个立体中心和一个新的碳-碳键,而双作用 NapH1 催化氯化和醚化反应在路径的两个不同阶段。此外,我们使用重组萘放射霉素生物合成酶在 1 天内在一锅中化学酶法合成毫克量。这种方法代表了一种可行的对映选择性方法来生产复杂的卤代代谢物,如尚未化学合成的萘二甲酸 B1。双作用 NapH1 在该途径的两个不同阶段催化氯化和醚化反应。此外,我们使用重组萘放射霉素生物合成酶在 1 天内在一锅中化学酶法合成毫克量。这种方法代表了一种可行的对映选择性方法来生产复杂的卤代代谢物,如尚未化学合成的萘二甲酸 B1。双作用 NapH1 在该途径的两个不同阶段催化氯化和醚化反应。此外,我们使用重组萘放射霉素生物合成酶在 1 天内在一锅中化学酶法合成毫克量。这种方法代表了一种可行的对映选择性方法来生产复杂的卤代代谢物,如尚未化学合成的萘二甲酸 B1。






































 京公网安备 11010802027423号
京公网安备 11010802027423号