当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Design of Versatile Biosensors for Small Molecules Based on the PAS Domain of a Thermophilic Histidine Kinase
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-12-10 00:00:00 , DOI: 10.1021/acssynbio.8b00348 Kai U. Cormann 1 , Meike Baumgart 1 , Michael Bott 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-12-10 00:00:00 , DOI: 10.1021/acssynbio.8b00348 Kai U. Cormann 1 , Meike Baumgart 1 , Michael Bott 1
Affiliation
|
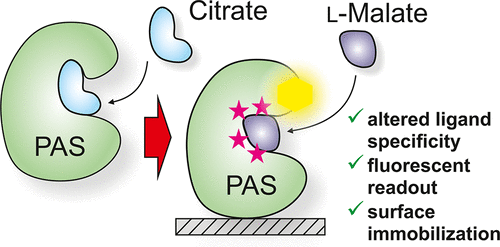
The development of biosensors for in vitro quantification of small molecules such as metabolites or man-made chemicals is still a major challenge. Here we show that engineered variants of the sensory PAS domain of the histidine kinase CitA of the thermophilic bacterium Geobacillus thermoleovorans represent promising alternatives to established biorecognition elements. By combining binding site grafting and rational design we constructed protein variants binding l-malate, ethylmalonate, or the aromatic compound phthalate instead of the native ligand citrate. Due to more favorable entropy contributions, the wild-type protein and its engineered variants exhibited increased (nano- to micromolar) affinities and improved enantioselectivity compared to CitA homologues of mesophilic organisms. Ligand binding was directly converted into an optical signal that was preserved after immobilization of the protein. A fluorescently labeled variant was used to quantify ethylmalonate, an urinary biomarker for ethylmalonic encephalopathy, in synthetic urine, thereby demonstrating the applicability of the sensor in complex samples.
中文翻译:

基于结构化嗜热组氨酸激酶PAS域的小分子多功能生物传感器的基于结构的设计。
开发用于体外定量小分子(例如代谢产物或人造化学物质)的生物传感器仍然是一项重大挑战。在这里我们显示,嗜热细菌热芽孢杆菌组氨酸激酶CitA的感觉PAS结构域的工程变体代表已建立的生物识别元件的有希望的替代品。通过结合结合位点嫁接和合理的设计,我们构造蛋白变体结合升-苹果酸酯,丙二酸乙酯或芳族化合物邻苯二甲酸酯,而不是天然的配体柠檬酸酯。由于更有利的熵贡献,与中温生物的CitA同系物相比,野生型蛋白及其工程变体表现出增加的(纳米至微摩尔)亲和力和改进的对映选择性。配体结合被直接转换为光信号,该信号在固定蛋白质后得以保留。使用荧光标记的变体对合成尿中乙基丙二酸脑病的尿生物标志物丙二酸乙酯进行定量,从而证明传感器在复杂样品中的适用性。
更新日期:2018-12-10
中文翻译:

基于结构化嗜热组氨酸激酶PAS域的小分子多功能生物传感器的基于结构的设计。
开发用于体外定量小分子(例如代谢产物或人造化学物质)的生物传感器仍然是一项重大挑战。在这里我们显示,嗜热细菌热芽孢杆菌组氨酸激酶CitA的感觉PAS结构域的工程变体代表已建立的生物识别元件的有希望的替代品。通过结合结合位点嫁接和合理的设计,我们构造蛋白变体结合升-苹果酸酯,丙二酸乙酯或芳族化合物邻苯二甲酸酯,而不是天然的配体柠檬酸酯。由于更有利的熵贡献,与中温生物的CitA同系物相比,野生型蛋白及其工程变体表现出增加的(纳米至微摩尔)亲和力和改进的对映选择性。配体结合被直接转换为光信号,该信号在固定蛋白质后得以保留。使用荧光标记的变体对合成尿中乙基丙二酸脑病的尿生物标志物丙二酸乙酯进行定量,从而证明传感器在复杂样品中的适用性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号