当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reducing Limitation in Probe Design: The Development of a Diazirine-Compatible Suzuki-Miyaura Cross Coupling Reaction.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-12-06 , DOI: 10.1021/acsmedchemlett.8b00403 Naoko Ichiishi 1 , Keith P Moore 2 , Anne Mai Wassermann 3 , Scott E Wolkenberg 2 , Shane W Krska 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-12-06 , DOI: 10.1021/acsmedchemlett.8b00403 Naoko Ichiishi 1 , Keith P Moore 2 , Anne Mai Wassermann 3 , Scott E Wolkenberg 2 , Shane W Krska 1
Affiliation
|
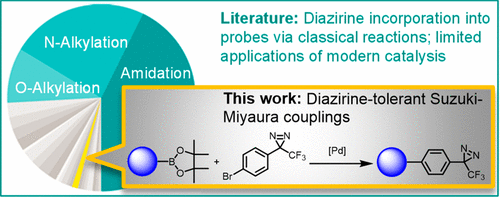
Access to high quality photoaffinity probe molecules is often constrained by synthetic limitations related to diazirine installation. A survey of recently published photoaffinity probe syntheses identified the Suzuki-Miyaura (S-M) coupling reaction, ubiquitous in drug discovery, as being underutilized to incorporate diazirines. To test whether advances in modern cross-coupling catalysis might enable efficient S-M couplings tolerant of the diazirine moiety, a fragment-based screening approach was employed. A model S-M coupling reaction was screened under various conditions in the presence of an aromatic diazirine fragment. This screen identified reaction conditions that gave good yields of S-M coupling product while minimally perturbing the diazirine reporter fragment. These conditions were found to be highly scalable and exhibited broad scope when applied to a chemistry informer library of 24 pharmaceutically relevant aryl boron pinacol esters. Furthermore, these conditions were used to synthesize a known diazirine-containing probe molecule with improved synthetic efficiency.
中文翻译:

减少探针设计的局限性:兼容Diazirine的Suzuki-Miyaura交叉偶联反应的发展。
获得高质量的光亲和探针分子通常受到与二嗪胺安装相关的合成限制的限制。对最近发表的光亲和探针合成的一项调查发现,在药物开发中普遍存在的Suzuki-Miyaura(SM)偶联反应未充分利用来掺入重氮。为了测试现代交叉偶联催化技术的进展是否可能使二叠氮基部分具有高效的SM偶联耐受性,采用了基于片段的筛选方法。在各种条件下,在芳族二嗪胺片段的存在下,筛选SM耦合反应模型。该筛查确定了反应条件,该条件给出了SM偶联产物的良好收率,同时最小化了对二嗪基报道片段的干扰。当将这些条件应用于24种药学上相关的芳基硼频哪醇酯的化学信息提供者库时,发现这些条件具有较高的可扩展性并显示出广泛的范围。此外,这些条件用于合成已知的含重氮基的探针分子,具有提高的合成效率。
更新日期:2018-12-06
中文翻译:

减少探针设计的局限性:兼容Diazirine的Suzuki-Miyaura交叉偶联反应的发展。
获得高质量的光亲和探针分子通常受到与二嗪胺安装相关的合成限制的限制。对最近发表的光亲和探针合成的一项调查发现,在药物开发中普遍存在的Suzuki-Miyaura(SM)偶联反应未充分利用来掺入重氮。为了测试现代交叉偶联催化技术的进展是否可能使二叠氮基部分具有高效的SM偶联耐受性,采用了基于片段的筛选方法。在各种条件下,在芳族二嗪胺片段的存在下,筛选SM耦合反应模型。该筛查确定了反应条件,该条件给出了SM偶联产物的良好收率,同时最小化了对二嗪基报道片段的干扰。当将这些条件应用于24种药学上相关的芳基硼频哪醇酯的化学信息提供者库时,发现这些条件具有较高的可扩展性并显示出广泛的范围。此外,这些条件用于合成已知的含重氮基的探针分子,具有提高的合成效率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号