当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifunctional Biphenyl-2-ylphosphine Ligand Enables Tandem Gold-Catalyzed Propargylation of Aldehyde and Unexpected Cycloisomerization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-12-10 , DOI: 10.1021/jacs.8b12478 Ting Li 1, 2 , Liming Zhang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-12-10 , DOI: 10.1021/jacs.8b12478 Ting Li 1, 2 , Liming Zhang 1
Affiliation
|
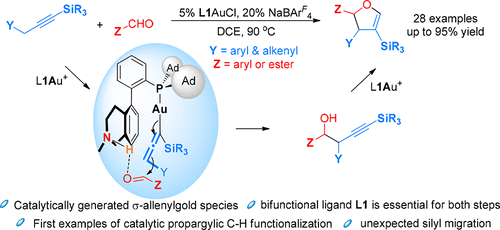
Despite extensive studies in gold catalysis, σ-allenylgold species have not been invoked as catalytic intermediates and their reactivities not studied. This work reports for the first time they are generated in situ and undergo nucleophilic addition to activated aldehydes in a bifunctional phosphine ligand-enabled gold catalysis. This development reveals a broad range of opportunities to achieve propargylic C-H functionalization for the first time under catalytic and mild conditions. The homopropargylic alcohols generated undergo ligand-enabled cycloisomerizations involving an unexpected silyl migration.
中文翻译:

双功能联苯-2-基膦配体使醛的串联金催化丙炔化和意外的环异构化成为可能
尽管对金催化进行了广泛的研究,但尚未将 σ-烯丙基金物种用作催化中间体,也未研究它们的反应性。这项工作首次报道了它们是原位生成的,并在双功能膦配体启用的金催化中对活化的醛进行亲核加成。这一发展揭示了在催化和温和条件下首次实现炔丙基 CH 官能化的广泛机会。生成的高炔丙醇经历配体启用的环异构化,涉及意外的甲硅烷基迁移。
更新日期:2018-12-10
中文翻译:

双功能联苯-2-基膦配体使醛的串联金催化丙炔化和意外的环异构化成为可能
尽管对金催化进行了广泛的研究,但尚未将 σ-烯丙基金物种用作催化中间体,也未研究它们的反应性。这项工作首次报道了它们是原位生成的,并在双功能膦配体启用的金催化中对活化的醛进行亲核加成。这一发展揭示了在催化和温和条件下首次实现炔丙基 CH 官能化的广泛机会。生成的高炔丙醇经历配体启用的环异构化,涉及意外的甲硅烷基迁移。






























 京公网安备 11010802027423号
京公网安备 11010802027423号