当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Critical Considerations on the Clinical Translation of Upconversion Nanoparticles (UCNPs): Recommendations from the European Upconversion Network (COST Action CM1403).
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-12-10 , DOI: 10.1002/adhm.201801233 Helena Oliveira 1 , Artur Bednarkiewicz 2, 3 , Andreas Falk 4 , Eleonore Fröhlich 5 , Darja Lisjak 6 , Adriele Prina-Mello 7 , Susanne Resch 4 , Christa Schimpel 4 , Ivana Vinković Vrček 8 , Edyta Wysokińska 9 , Hans H Gorris 10
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-12-10 , DOI: 10.1002/adhm.201801233 Helena Oliveira 1 , Artur Bednarkiewicz 2, 3 , Andreas Falk 4 , Eleonore Fröhlich 5 , Darja Lisjak 6 , Adriele Prina-Mello 7 , Susanne Resch 4 , Christa Schimpel 4 , Ivana Vinković Vrček 8 , Edyta Wysokińska 9 , Hans H Gorris 10
Affiliation
|
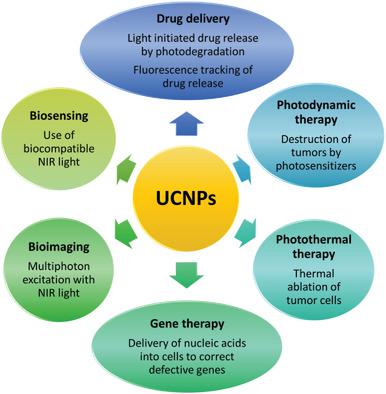
The unique photoluminescent properties of upconversion nanoparticles (UCNPs) have attracted worldwide research interest and inspired many bioanalytical applications. The anti-Stokes emission with long luminescence lifetimes, narrow and multiple absorption and emission bands, and excellent photostability enable background-free and multiplexed detection in deep tissues. So far, however, in vitro and in vivo applications of UCNPs are restricted to the laboratory use due to safety concerns. Possible harmful effects may originate from the chemical composition but also from the small size of UCNPs. Potential end users must rely on well-founded safety data. Thus, a risk to benefit assessment of the envisioned combined therapeutic and diagnostic ("theranostic") applications is fundamentally important to bridge the translational gap between laboratory and clinics. The COST Action CM1403 "The European Upconversion Network-From the Design of Photon-Upconverting Nanomaterials to Biomedical Applications" integrates research on UCNPs ranging from fundamental materials synthesis and research, detection instrumentation, biofunctionalization, and bioassay development to toxicity testing. Such an interdisciplinary approach is necessary for a better and safer theranostic use of UCNPs. Here, the status of nanotoxicity research on UCNPs is compared to other nanomaterials, and routes for the translation of UCNPs into clinical applications are delineated.
中文翻译:

上转换纳米粒子(UCNPs)的临床翻译的关键考虑因素:欧洲上转换网络(COST Action CM1403)的建议。
上转换纳米颗粒(UCNPs)的独特光致发光特性吸引了全世界的研究兴趣,并激发了许多生物分析应用。具有长发光寿命,窄和多个吸收和发射带以及出色的光稳定性的抗斯托克斯发射特性使得能够在深部组织中实现无背景和多重检测。但是,到目前为止,出于安全考虑,UCNP的体外和体内应用仅限于实验室使用。可能的有害影响可能源自化学成分,也可能源自较小的UCNP。潜在的最终用户必须依靠有充分根据的安全数据。因此,对设想的组合治疗和诊断(“热疗” )应用对于弥合实验室和诊所之间的翻译鸿沟至关重要。COST行动CM1403“欧洲上转换网络-从光子上转换纳米材料的设计到生物医学应用”整合了UCNP的研究,从基础材料的合成和研究,检测仪器,生物功能化,生物测定开发到毒性测试。这种跨学科的方法对于更好,更安全地治疗UCNPs是必要的。在这里,将UCNPs的纳米毒性研究现状与其他纳米材料进行了比较,并描述了UCNPs转化为临床应用的途径。
更新日期:2018-12-10
中文翻译:

上转换纳米粒子(UCNPs)的临床翻译的关键考虑因素:欧洲上转换网络(COST Action CM1403)的建议。
上转换纳米颗粒(UCNPs)的独特光致发光特性吸引了全世界的研究兴趣,并激发了许多生物分析应用。具有长发光寿命,窄和多个吸收和发射带以及出色的光稳定性的抗斯托克斯发射特性使得能够在深部组织中实现无背景和多重检测。但是,到目前为止,出于安全考虑,UCNP的体外和体内应用仅限于实验室使用。可能的有害影响可能源自化学成分,也可能源自较小的UCNP。潜在的最终用户必须依靠有充分根据的安全数据。因此,对设想的组合治疗和诊断(“热疗” )应用对于弥合实验室和诊所之间的翻译鸿沟至关重要。COST行动CM1403“欧洲上转换网络-从光子上转换纳米材料的设计到生物医学应用”整合了UCNP的研究,从基础材料的合成和研究,检测仪器,生物功能化,生物测定开发到毒性测试。这种跨学科的方法对于更好,更安全地治疗UCNPs是必要的。在这里,将UCNPs的纳米毒性研究现状与其他纳米材料进行了比较,并描述了UCNPs转化为临床应用的途径。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号