当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mapping Aldehyde Dehydrogenase 1A1 Activity using an [18F]Substrate‐Based Approach
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-01-14 , DOI: 10.1002/chem.201805473 Raul Pereira 1, 2 , Thibault Gendron 3 , Chandan Sanghera 1, 2 , Hannah E Greenwood 1, 2 , Joseph Newcombe 3, 4 , Patrick N McCormick 1, 2 , Kerstin Sander 3 , Maya Topf 4 , Erik Årstad 3 , Timothy H Witney 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-01-14 , DOI: 10.1002/chem.201805473 Raul Pereira 1, 2 , Thibault Gendron 3 , Chandan Sanghera 1, 2 , Hannah E Greenwood 1, 2 , Joseph Newcombe 3, 4 , Patrick N McCormick 1, 2 , Kerstin Sander 3 , Maya Topf 4 , Erik Årstad 3 , Timothy H Witney 1, 2
Affiliation
|
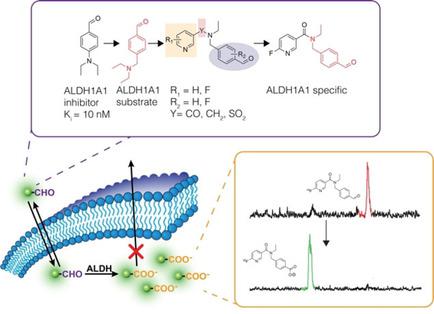
Aldehyde dehydrogenases (ALDHs) catalyze the oxidation of aldehydes to carboxylic acids. Elevated ALDH expression in human cancers is linked to metastases and poor overall survival. Despite ALDH being a poor prognostic factor, the non‐invasive assessment of ALDH activity in vivo has not been possible due to a lack of sensitive and translational imaging agents. Presented in this report are the synthesis and biological evaluation of ALDH1A1‐selective chemical probes composed of an aromatic aldehyde derived from N,N‐diethylamino benzaldehyde (DEAB) linked to a fluorinated pyridine ring either via an amide or amine linkage. Of the focused library of compounds evaluated, N‐ethyl‐6‐(fluoro)‐N‐(4‐formylbenzyl)nicotinamide 4 b was found to have excellent affinity and isozyme selectivity for ALDH1A1 in vitro. Following 18F‐fluorination, [18F]4 b was taken up by colorectal tumor cells and trapped through the conversion to its 18F‐labeled carboxylate product under the action of ALDH. In vivo positron emission tomography revealed high uptake of [18F]4 b in the lungs and liver, with radioactivity cleared through the urinary tract. Oxidation of [18F]4 b, however, was observed in vivo, which may limit the tissue penetration of this first‐in‐class radiotracer.
中文翻译:

使用基于 [18F] 底物的方法绘制乙醛脱氢酶 1A1 活性图
醛脱氢酶(ALDH)催化醛氧化成羧酸。人类癌症中 ALDH 表达升高与转移和总体生存率较差有关。尽管 ALDH 是一个不良的预后因素,但由于缺乏敏感的转化成像剂,对体内 ALDH 活性的非侵入性评估还不可能。本报告介绍了 ALDH1A1 选择性化学探针的合成和生物学评价,该探针由衍生自N , N-二乙氨基苯甲醛 (DEAB) 的芳香醛组成,通过酰胺键或胺键与氟化吡啶环连接。在所评估的重点化合物库中,发现N-乙基-6-(氟) -N- (4-甲酰基苄基)烟酰胺4b在体外对 ALDH1A1 具有优异的亲和力和同工酶选择性。 18 F-氟化后,[ 18 F] 4 b被结直肠肿瘤细胞摄取,并在 ALDH 的作用下通过转化为其18 F 标记的羧酸盐产物而被捕获。体内正电子发射断层扫描显示肺和肝脏对[ 18 F] 4 b的高摄取,放射性通过尿路清除。然而,在体内观察到[ 18 F] 4 b的氧化,这可能会限制这种一流的放射性示踪剂的组织渗透。
更新日期:2019-01-14
中文翻译:

使用基于 [18F] 底物的方法绘制乙醛脱氢酶 1A1 活性图
醛脱氢酶(ALDH)催化醛氧化成羧酸。人类癌症中 ALDH 表达升高与转移和总体生存率较差有关。尽管 ALDH 是一个不良的预后因素,但由于缺乏敏感的转化成像剂,对体内 ALDH 活性的非侵入性评估还不可能。本报告介绍了 ALDH1A1 选择性化学探针的合成和生物学评价,该探针由衍生自N , N-二乙氨基苯甲醛 (DEAB) 的芳香醛组成,通过酰胺键或胺键与氟化吡啶环连接。在所评估的重点化合物库中,发现N-乙基-6-(氟) -N- (4-甲酰基苄基)烟酰胺4b在体外对 ALDH1A1 具有优异的亲和力和同工酶选择性。 18 F-氟化后,[ 18 F] 4 b被结直肠肿瘤细胞摄取,并在 ALDH 的作用下通过转化为其18 F 标记的羧酸盐产物而被捕获。体内正电子发射断层扫描显示肺和肝脏对[ 18 F] 4 b的高摄取,放射性通过尿路清除。然而,在体内观察到[ 18 F] 4 b的氧化,这可能会限制这种一流的放射性示踪剂的组织渗透。






























 京公网安备 11010802027423号
京公网安备 11010802027423号