Synthesis ( IF 2.2 ) Pub Date : 2018-12-06 , DOI: 10.1055/s-0037-1610391 Samuel Bartlett , Kimberly Keiter , Blane Zavesky , Jeffrey Johnson 1
|
This work was carried out in the Department of Chemistry at UNC Chapel Hill, which celebrated its bicentennial in 2018.
Published as part of the 50 Years SYNTHESIS – Golden Anniversary Issue
Abstract
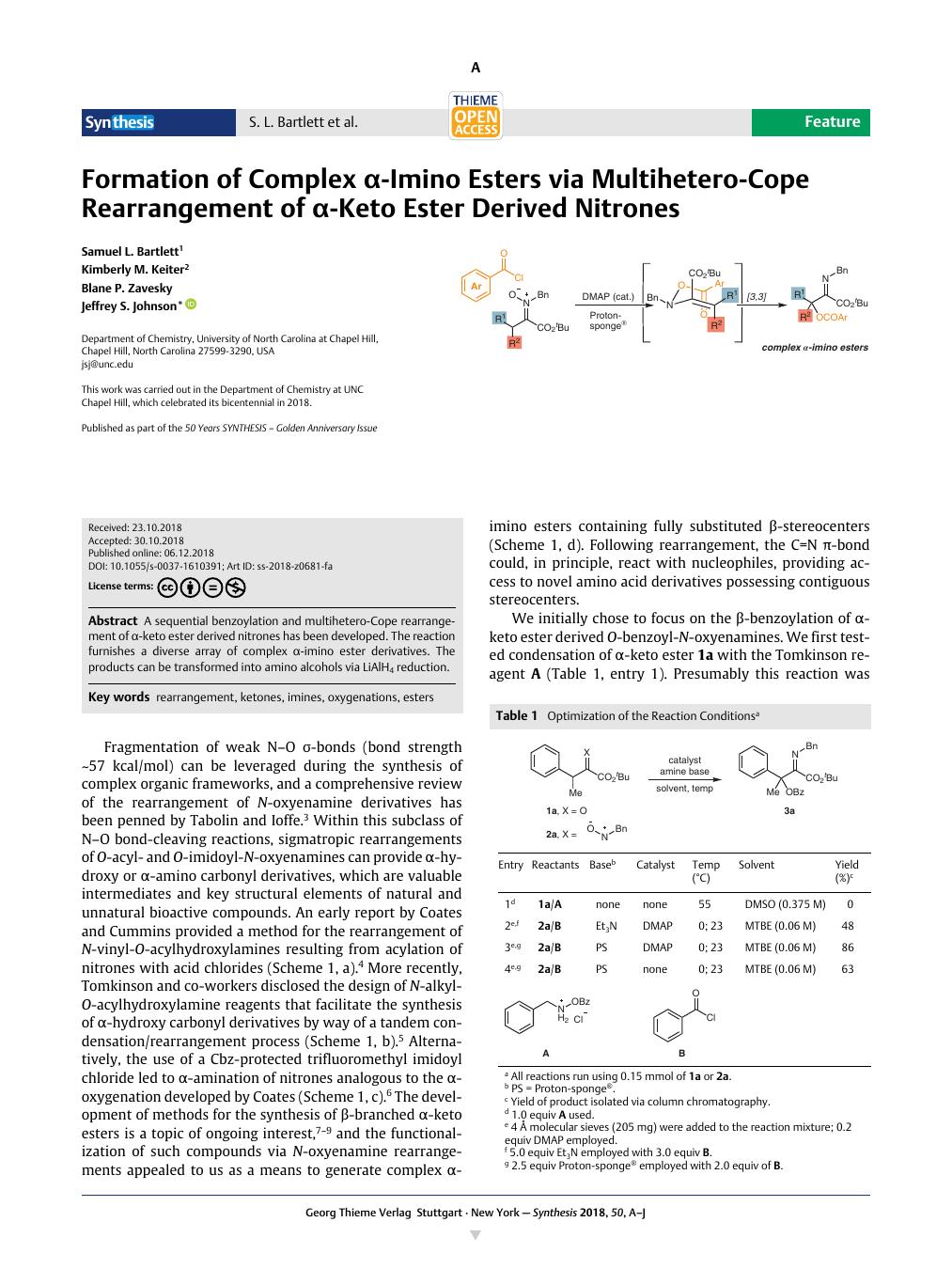
A sequential benzoylation and multihetero-Cope rearrangement of α-keto ester derived nitrones has been developed. The reaction furnishes a diverse array of complex α-imino ester derivatives. The products can be transformed into amino alcohols via LiAlH4 reduction.
A sequential benzoylation and multihetero-Cope rearrangement of α-keto ester derived nitrones has been developed. The reaction furnishes a diverse array of complex α-imino ester derivatives. The products can be transformed into amino alcohols via LiAlH4 reduction.
中文翻译:

通过α-酮基酯衍生的多价多杂应对的重排形成复杂的α-氨基酯
这项工作是在UNC教堂山的化学系进行的,该系于2018年庆祝了其诞辰200周年。
作为《五十周年综合报告》的一部分发行-黄金周年纪念日
抽象的
已经开发出α-酮基酯衍生的硝酮的顺序苯甲酰化和多杂-Cope重排。该反应提供了各种各样的复杂的α-亚氨基酯衍生物。可以通过LiAlH 4还原将产物转化为氨基醇。
已经开发出α-酮基酯衍生的硝酮的顺序苯甲酰化和多杂-Cope重排。该反应提供了各种各样的复杂的α-亚氨基酯衍生物。可以通过LiAlH 4还原将产物转化为氨基醇。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号